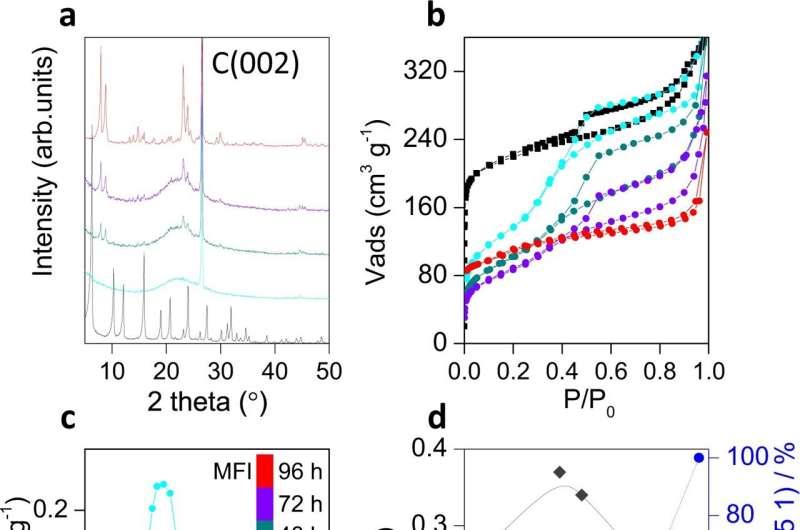
Crystallinity and textural evolution. a XRD patterns of solids obtained at different times during the interzeolite transformation of FAU zeolite into MFI using CTPABr as both SDA and porogen (see treatment time in c). The strong band at ca. 26° 2θ corresponds to the graphite used as internal standard. b N2 physisorption isotherms at 77 K for the same samples. c The corresponding pore size distribution calculated by NL-DFT from the isotherms. d Evolution of the micropore volume (green triangles), mesopore volume (gray diamonds), and MFI crystallinity (blue circles) of the samples with time. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-36502-3
The Laboratory of Molecular Nanotechnology (NANOMOL) of the University of Alicante (UA) has developed a new family of materials as a state-of-the-art opportunity for the chemical industry, renewables, and the reduction of pollutants. The finding, published in the journal Nature Communications, opens up numerous possibilities for sectors such as energy and pharmaceuticals.
In general, scientists are looking to make highly ordered materials. For example, zeolites, the most important and widely used family of catalysts in the chemical industry, are made of periodically repeating units. According to Noemí Linares, UA researcher and author of the paper, scientists have moved away from materials that are somewhere between messy and orderly, where the opportunities are endless. They have realized that in the imperfect and unordered materials, there are countless opportunities to make new materials with unique properties.
In the defective and disordered nature, there are not the limitations often imposed by regular structures, which opens up endless opportunities for the creation and design of materials, as explained by Javier García Martínez, professor of Inorganic Chemistry at the UA and director of NANOMOL. Based on this idea, UA researchers have built materials that are halfway between ordered structures, called zeolites.
These hybrid materials have important advantages such as a high surface area, which allows them to transform very bulky molecules, something that was not possible with conventional zeolites so far, which have very narrow pores. In particular, it is on the borderline between ordered and disordered material, which has irregular but very large cavities that allow more complex and bulky molecules to be transformed as defined by the researcher and author of the article, Mónica J. Mendoza Castro.
In order to obtain these materials, the University of Alicante team of researchers has used a well-known process that allows one zeolite to be transformed into another, but interrupting this conversion to obtain intermediate half-made materials, which contain characteristics of both solids. UA professor explains the process as if they had stopped the transformation of a worm into a butterfly, when it is not yet complete, and at that point they have discovered that at that stage there is something completely new, fascinating and with innumerable applications.
The finding presents a revolution in the field of catalysis that is key to making the chemical industry more sustainable. In this case, as reported by NoemÍ Linares in the article published in Nature Communications, they selected the most interesting parts of each zeolite to make something new and with the most suitable composition for each application. Additionally, the structural flexibility of the materials designed by the UA allows the molecules to enter and exit more easily, reducing carbon waste and CO2 emissions into the atmosphere.
More information: Monica J. Mendoza-Castro et al, Tunable hybrid zeolites prepared by partial interconversion, Nature Communications (2023). DOI: 10.1038/s41467-023-36502-3
Journal information: Nature Communications
Provided by Asociacion RUVID