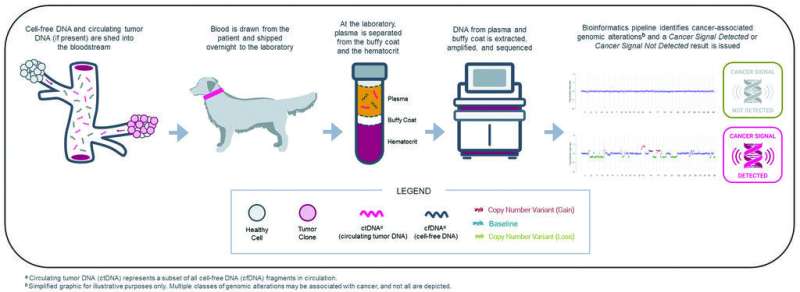
Visual representation of the steps involved in next-generation sequencing–based liquid biopsy testing, from cell-free DNA entering the bloodstream through bioinformatics analysis at the laboratory. Credit: Journal of the American Veterinary Medical Association (2023). DOI: 10.2460/javma.22.11.0526
PetDx published a study today showing that OncoK9, its multi-cancer early detection test for dogs using next-generation sequencing (NGS) technology, performs similarly in real-world veterinary practice settings as in the CANcer Detection in Dogs (CANDiD) study, the test's landmark clinical validation study.
Appearing in the Journal of the American Veterinary Medical Association, the new study examined the clinical experience with 1,500 consecutive blood samples submitted to PetDx in early 2022 for commercial testing where the patient's cancer status was unknown at the time of the blood collection.
The new study found that patients who received a "Cancer Signal Detected" (positive) result from the OncoK9 test subsequently received a clinical cancer diagnosis from a veterinarian in nearly 90% of cases. And in the vast majority of these cases, the diagnosis was achieved within two weeks using tools commonly available in the ordering veterinarian's clinic.
"This study in JAVMA clearly demonstrates that NGS-based liquid biopsy enables veterinarians to detect cancer in dogs with a simple blood draw," said lead author and PetDx Director of Clinical Support Allison O'Kell, DVM, MS, DACVIM (Small Animal Internal Medicine).
"Through our published studies, we have built up the evidence base to confidently show that OncoK9 works and provides value in the real world. Veterinarians are data-driven clinicians, and studies that demonstrate how a test performs in the postmarketing setting are very important to inform clinical decision-making related to the adoption of novel technologies in day-to-day practice."
When used as a screening test in high-risk dogs without clinical signs (64% of cases in the study), OncoK9 detected 26 types of cancer in patients, half of which are not commonly detected on wellness exams (such as cancers of the spleen, liver, and lung). This observation suggests that adding OncoK9 to wellness visits may help to expand the number of cancer types and cancer cases that can be detected preclinically.
Among patients that had the OncoK9 test for cancer screening and received a negative result, cancer was not found and was not clinically suspected, by the time the study outcome collection closed, in over 94% of cases—providing peace of mind to the dogs' veterinarians and families.
This study is the latest in a series of synergistic scientific publications by PetDx since 2021, establishing the value of NGS-based liquid biopsy testing for cancer detection in dogs:
- A comprehensive review article on cancer genomics and liquid biopsy applications in veterinary medicine. (Frontiers in Veterinary Science, March 2021)
- A proof-of-concept study establishing the feasibility of using cell-free DNA with NGS for canine cancer detection. (Frontiers in Veterinary Science, July 2021)
- The CANcer Detection in Dogs (CANDiD) study, the largest clinical validation study ever performed in veterinary cancer diagnostics. (PLOS ONE, April 2022)
- A study indicating that adding NGS-based liquid biopsy to a dog's wellness visit can help increase early cancer detection. (Journal of Veterinary Internal Medicine, January 2023)
- A study defining the optimal age to start cancer screening in dogs. (PLOS ONE, February 2023)
"This series of groundbreaking peer-reviewed studies—from proof of concept and clinical validation to this latest evaluation of OncoK9 in real-world conditions—represents an unmatched commitment by PetDx to science-based excellence and underscores our leadership in veterinary cancer diagnostics," said PetDx President and CEO Alejandro Bernal, DVM, MS, MBA.
"Not only do we expect this level of transparency from ourselves, but we also encourage veterinarians to demand it from all laboratories offering solutions for early cancer detection in pets. Veterinarians and their patients deserve high-quality results, and PetDx is proud to deliver on this expectation with published world-class research."
More information: Allison L. O'Kell et al, Clinical experience with next-generation sequencing–based liquid biopsy testing for cancer detection in dogs: a review of 1,500 consecutive clinical cases, Journal of the American Veterinary Medical Association (2023). DOI: 10.2460/javma.22.11.0526
Journal information: Journal of the American Veterinary Medical Association , PLoS ONE
Provided by Stephens & Associates