Credit: The Francis Crick Institute
Head of the Crick's Stem Cells and Neuromuscular Regeneration Lab, Francesco Saverio Tedesco, is also a doctor at Great Ormond Street Hospital, where he specializes in neurological and neuromuscular diseases of childhood, like muscular dystrophy. In his lab, his team are working on brand new ways to generate muscle tissue, that will help researchers understand diseases where muscles deteriorate, but could also be the first steps towards future muscle regeneration therapies.
The team have published their latest methods for growing skeletal muscle tissue from stem cells, in Nature Protocols, and we spoke to Saverio about their progress.
What are the challenges of growing muscle tissue?
We've entered into a new era of regenerative medicine. The advanced tools at our disposal today mean that it's now possible to grow incredibly complex structures in the lab. But there are still challenges. It's a time and resource-intensive process and the conditions have to be just right.
How have your team approached muscle generation?
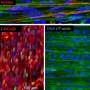
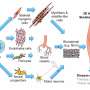
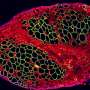
We have taken a 3D bioengineering approach. We start with human induced pluripotent stem cells, which are capable of becoming any cell type in the body. We then grew these into the different cell types that make up skeletal muscle—myogenic (muscle) cells, neurons, and vascular cells for the growth of blood vessels. We combined these within 3D hydrogels under tension to create a scaffold in which the muscle would grow.
The end result is a small piece of skeletal muscle tissue, about 1cm in size. And the interesting thing about this method is that we can create lab-grown muscles that are reflective of specific patients. The induced pluripotent stem cells can be grown from a patient's own skin cells and the resulting muscle structure will reflect features of the muscle disease they have.
Contractions of hiPSC-derived 3D skeletal muscle constructs. Credit: The Francis Crick Institute
How long does it take to grow muscle tissue?
The length of time varies depending on the complexity and size of the end structure. If the muscle-making cells are readily available, the mini muscles can be produced in about two weeks. But it could take up to 3-4 months if you start from scratch, i.e. if one has to generate also the induced pluripotent stem cells. Creating and differentiating the induced pluripotent stem cells take around two months and then it takes a further month to grow them together in the hydrogel to create the tissue. It's not a quick process, but now we have a bank of frozen stem cells that we can create muscle from in just a few weeks.
How are you planning to use the lab-grown muscles?
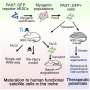
Because we can grow muscle from specific patients, we can use these structures to better understand the biology of different conditions characterized by muscle deterioration. We can also use the lab-grown muscle to test potential new treatments. In our Nature Protocols paper, we showed that the structures are responsive to inactive viruses used for new gene therapies in a dose-dependent manner. We also showed that mini muscles could be used to study how stem cells behave when delivered to muscle tissue, providing a tool to optimize possible future cell transplantation strategies for muscle diseases.
How could muscle generation be used in future treatment?
I think a clear use for artificial muscles will be in finding and testing new treatments for diseases like muscular dystrophy. The muscles could also be grown from individual patients to help personalize their care by selecting treatments that are most likely to work based on how well the artificial muscle responds.
But one day we might also be able to grow and transplant sections of muscle. In our paper we also showed that the artificial muscle can be engrafted and implanted in mice as a form of tissue replacement therapy.
More information: Luca Pinton et al, 3D human induced pluripotent stem cell–derived bioengineered skeletal muscles for tissue, disease and therapy modeling, Nature Protocols (2023). DOI: 10.1038/s41596-022-00790-8
Journal information: Nature Protocols
Provided by The Francis Crick Institute