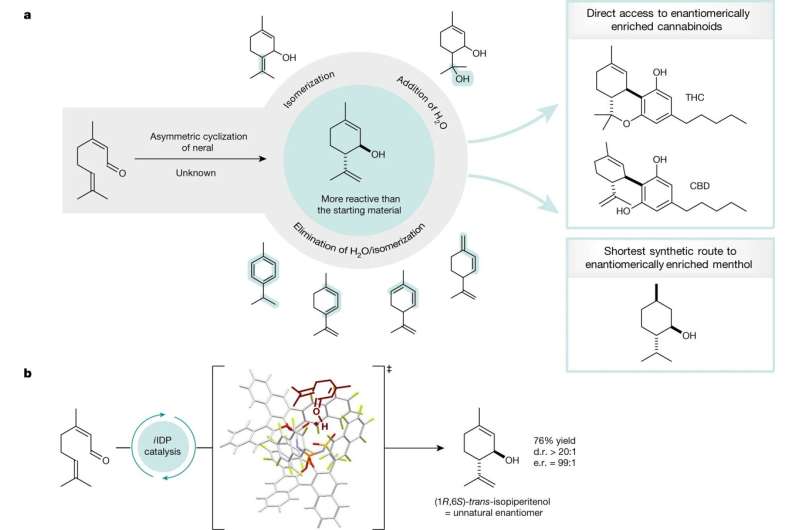
Brønsted acid-catalyzed cyclization of neral to (1R,6S)-trans-isopiperitenol. a, Selectivity problems in the cyclization of neral named the ‘neral to isopiperitenol dilemma’ and isopiperitenol as valuable chiral pool material in the synthesis of cannabinoids and menthol. b, The first asymmetric, selective cyclization of neral to isopiperitenol under confined Brønsted acid catalysis. Credit: Nature (2023). DOI: 10.1038/s41586-023-05747-9
Menthol is a substance that exists naturally in various mint plants. For hundreds of years, humans have recognized it as useful, causing an immense demand for menthol worldwide. Moreover, extraction from the plants itself is costly. So each year, thousands of tons of menthol are being produced synthetically.
Benjamin List, director at the Max-Planck-Institut für Kohlenforschung in Mülheim, and his team have now succeeded in simplifying the synthetic production of menthol—making it cheaper and more sustainable. The same methodology can, by the way, be used to produce cannabinoids, also sought-after ingredients due to their medical applications. The Mülheim scientists have now published their results in Nature.
For many years, chemists have envisioned a "dream reaction" depicting a significant shortcut in the synthesis of menthol or cannabinoids. Specifically, it involves the selective conversion of neral to a particular molecule called isopiperitenol.
"For a long time, however, this particular reaction was considered impossible because the product was more reactive to the catalyst than the starting materials," explains Joyce Grimm. She is a doctoral student in the List group and responsible for this project. More than 100 years ago, chemists Albert Verley and Friedrich Wilhelm Semmler already had tried that reaction—unsuccessfully. Ben List, Joyce Grimm and their colleagues have now succeeded.
For their method, the Mülheim researchers use a certain confined acid as catalyst, as their "molecular tool." This asymmetric, strong acid helps to form the desired product with a high degree of efficiency and selectivity. The catalyst binds the product in an inert form so that no further, undesired side reaction can take place.
Using the same method, the Mülheim researchers have also succeeded in demonstrating how isopiperitenol can easily be converted into cannabinoids or menthol—in the shortest and most efficient way. This, again, is of great interest to the chemical industry because processes are shortened and chemical waste is avoided.
More information: Joyce A. A. Grimm et al, Catalytic asymmetric synthesis of cannabinoids and menthol from neral, Nature (2023). DOI: 10.1038/s41586-023-05747-9
Journal information: Nature
Provided by Max Planck Society