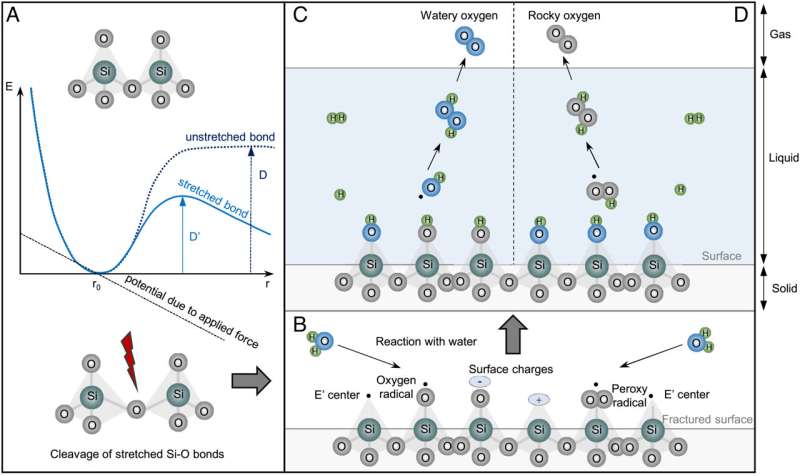
Two proposed mechanisms for H2O2 generation at quartz–water interfaces. (A) Stress-induced cleavage of Si–O bonds. Applied force is represented by a linear falling potential (dashed line), which is added to the Morse potential of the unstretched Si–O bond (dotted line), thus resulting in a force-deformed Morse potential (solid line). The new dissociation energy D′ is smaller than D in the case of a stretching force. Modified after Kauzmann et al. The probability of generation of surface-bound radicals in quartz therefore increases during mechanical processes. (B) Contact of water on freshly fractured surface of abraded quartz. Modified after Schoonen et al. (C) Generation of H2O2 and O2 in the reaction between H2O and oxygen radical (≡SiO•). (D) Generation of rocky H2O2 and O2 in the reaction between H2O and peroxy radical (≡SiOO•). Surface-bound radicals are formed through homolysis of Si–O bonds, including ≡SiO•, ≡SiOO•, and E′ center (≡Si•), while surface charges (≡Si+ and ≡SiO–) for heterolysis. Silanol groups (≡SiOH) are end products on quartz surface after hydroxylation. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2221984120
A team of geochemists from the Chinese Academy of Sciences, working with colleagues from the University of Hong Kong, Tianjin University and the University of California, has found evidence that suggests much of the oxygen in early Earth's early atmosphere may have come from rocks. In their study, reported in Proceedings of the National Academy of Sciences, the group conducted lab experiments involving crushing rocks, exposing the results to water and measuring reactive oxygen species that were emitted.
Prior research has shown that Earth experienced what has been called the Great Oxidation Event approximately 2.3 to 2.4 billion years ago. During this time, microbe numbers increased dramatically, as they released oxygen during photosynthesis. But prior research has also suggested that a common life ancestor existed before the Great Oxidation Event, which further suggests that there was some amount of oxygen exposure. In this new effort, the researchers suggest that such oxygen could have come from rocks interacting with water.
The work involved crushing samples of quartz and then exposing them to water, which replicates some of the conditions that existed on early Earth prior to the rise of high levels of oxygen in the atmosphere. Adding water to freshly crushed quartz, the researchers found, led to reactions between the water and newly broken crystals. This resulted it the formation of molecular oxygen along with other reactive oxygen species like hydrogen peroxide. Such species are also known as free radicals and they would have played an important role in the evolution of early life. This is because by damaging DNA and other cell components, the free radicals would have pressured early life to adapt.
A Mars-similar wind-erosion landform in Qiadam Basin, China. Credit: Xiao Wu
The researchers suggest a variety of events could have led to the release of such materials, such as earthquakes, erosion or the movement of glaciers. They further suggest that similar processes could be happening on other planets right now—with Mars sandstorms, for example, or fluctuating tides on moons that have water. Such processes, they point out, could produce oxygen, which could be play a role in the development of life.
More information: Hongping He et al, A mineral-based origin of Earth's initial hydrogen peroxide and molecular oxygen, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2221984120
Journal information: Proceedings of the National Academy of Sciences
© 2023 Science X Network