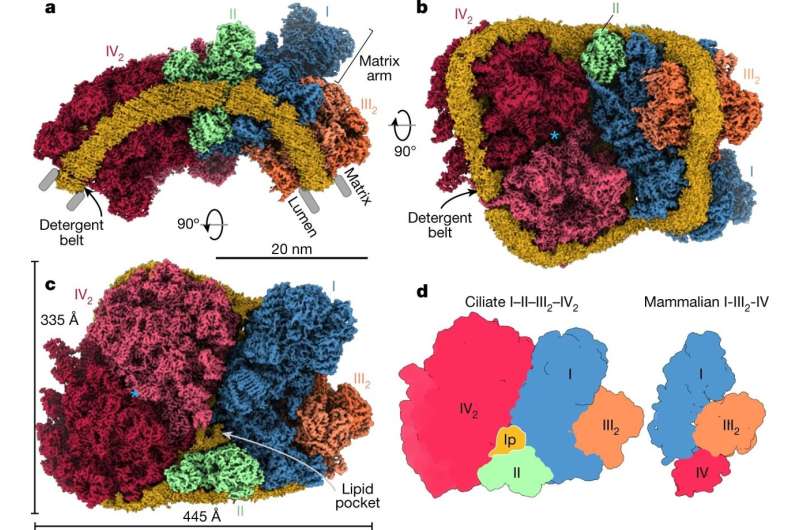
The supercomplex contains all four ETC components. a, Side view of the supercomplex density showing the curved detergent micelle (yellow). b, Lumenal view illustrates how complexes CI, CII and CIV2 stabilize each other. The blue asterisk indicates the symmetry axis of CIV2. c, Matrix view shows CII binding in a wedge between CI and CIV2, resulting in the enclosure of a lipid pocket (lp). The blue asterisk indicates the same position as in b. d, Architecture comparison of the ciliate I–II–III2–IV2 supercomplex (this study; left) with the mammalian I–III2–IV respirasome (Protein Data Bank (PDB) accession 5J4Z; right), highlighting a different location of CIV2 that is correlated with acquisition of CI subunits that stabilize CIII2.
Eukaryotes generate the energy for survival through cellular respiration in mitochondria by a process known as the oxidative phosphorylation. In this process, nutrients and oxygen are converted into a chemical form of energy: ATP. This is achieved with a proton gradient built up by the electron transport chain inside mitochondria.
The gradient is driven by a series of four respiratory complexes in the inner mitochondrial membrane. A new study published in Nature combined tomography and molecular simulations to shed light on bioenergetic macro-assemblies and how they shape mitochondrial membranes. It identified that in Tetrahymena thermophila—a free-living single cell eukaryote found in ponds and lakes—all four respiratory complexes are associated.
They form a massive 5.8 megadalton supercomplex of 150 proteins with at least 300 transmembrane helices and 311 lipids. Owing to subunit acquisition and extension, Complex I binds a dimer of Complex III that is tilted by 37 degrees. Complex I also associates with the Complex IV dimer, generating a gap that serves as a binding site for Complex II.
The study demonstrates that this assembly is crucial to the shaping of the bioenergetic membrane. One of the most intriguing findings is that a subunit of Complex IV called COX3 is split in two. The fragmentation occurs on the genetic level, and then each fragment is extended, contributing to some of the interfaces between complexes. The gain of function for inter-complex contacts represents an evolutionary mechanism, showing how neutral molecular complexity can become beneficial.
The evolution of protein subunits of respiratory complexes has led to the I–II–III2–IV2 assembly that contributes to the shaping of the bioenergetic membrane, thereby enabling its functional specialization. Credit: A. Amunts and Mad Microbe Studios
The findings highlight how the evolution of protein subunits of respiratory complexes has led to the supercomplex assembly, which actively contributes to mitochondrial membrane curvature induction that is necessary for proper mitochondrial function.
This way, the supercomplex shapes the macroscopic architecture of mitochondria, ultimately optimizing ATP synthesis. Therefore, respiratory supercomplexes have not only an enzymatic but also a structural function of shaping the membrane, and both together support energy conversion and provide fuel for life.
More information: Alexander Mühleip et al, Structural basis of mitochondrial membrane bending by the I–II–III2–IV2 supercomplex, Nature (2023). DOI: 10.1038/s41586-023-05817-y
Journal information: Nature
Provided by Science For Life Laboratory