This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biologists develop new record bright red fluorescent protein
To understand why a cell divides, secretes hormones or transmits a signal to another cell, biologists often use a trick. They attach colored lights to the proteins of interest, so that they can follow the movements and interactions of those proteins in living cells under the microscope. The more colors of these lights are available, the more processes they can follow at the same time.
In the 1990s, scientists used a fluorescent protein as a colored marker in a cell for the first time. That protein was green and came from a fluorescent jellyfish. By tinkering with that green protein, blue, turquoise and yellow variants followed. In the 2000s, a red fluorescent protein was discovered in corals. But turning this protein into a usable and bright red light for cell research proved to be a lot more challenging.
Creation of mScarlet3
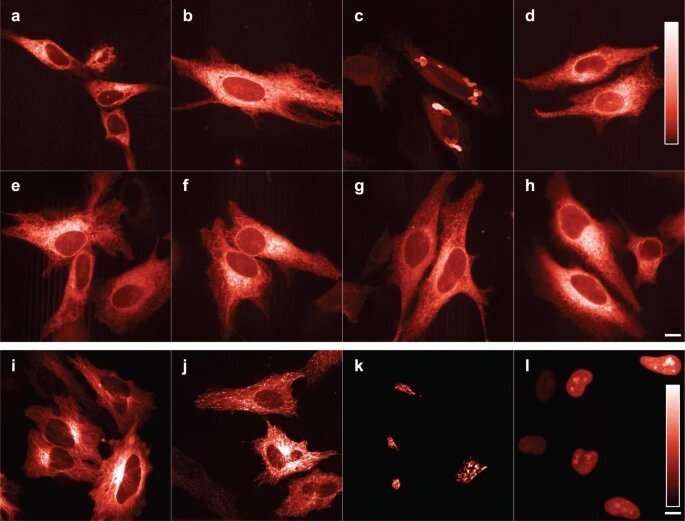
In 2016, the team of University of Amsterdam biologist Dorus Gadella succeeded in creating a new bright red fluorescent protein that formed a giant leap forward. They named that protein mScarlet. Their red glowing protein was quickly picked up by the scientific world. The DNA encoding mScarlet has been requested about 3,400 times and is now used for cell biology research in almost every country in the world.
Unfortunately, in mammalian cells the mScarlet protein turned out to fold more slowly and less completely than the commonly used green fluorescent proteins, causing the brightness to not be optimal in these cells. Therefore, the team continued working on the protein and tried to accelerate and maximize the folding.
They used two variants of mScarlet that they had already developed, one with fast folding but lower brightness and one with slow folding but ultimately bright fluorescence. They tried to combine the positive properties of these two into a new protein. With the help of a number of targeted changes in the structure of the protein, they managed to achieve this, resulting in mScarlet3. This newest variant now combines maximum brightness with fast and complete folding.
Finally, to test the structure of mScarlet3, the biologists sent their creation to the Institut de Biologie Structurale in Grenoble (CNRS, CEA, Université Grenoble Alpes). Structural biologist Antoine Royant used the European Synchrotron ESRF, the world's brightest X-ray source, to map the molecular structure of the protein.
Royant says, "It turned out that mScarlet3 is so bright because of a special hydrophobic (oily) local structure in the protein, which both speeds up and improves the folding of the protein."
The team's latest research is published in the journal Nature Methods.
New standard
With this new, much-improved version of the red fluorescent protein, the toolbox scientists have at their disposal in the lab is now more complete than ever. Gadella notes, "The experiences with mScarlet were already very positive, which is why we expect that mScarlet3 will become even more popular among researchers and will quickly become the new standard worldwide. Bright red fluorescent proteins are highly sought after because excitation of these red proteins is less harmful to cells than exciting green proteins.
"In addition, red light is scattered less, which means that you can also use the microscope to look at molecular processes in deeper cell layers. With mScarlet3 we finally have a very robust bright red fluorescent protein that folds quickly and completely without further disadvantages. We expect a lot from new applications with mScarlet3, including for making new red fluorescent biosensors where mScarlet3 can be used to image specific cell functions."
More information: Theodorus Gadella, mScarlet3: a brilliant and fast-maturing red fluorescent protein, Nature Methods (2023). DOI: 10.1038/s41592-023-01809-y
Journal information: Nature Methods
Provided by University of Amsterdam