This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers detect heavy oxygen isotope in Earth's upper mesosphere and lower thermosphere
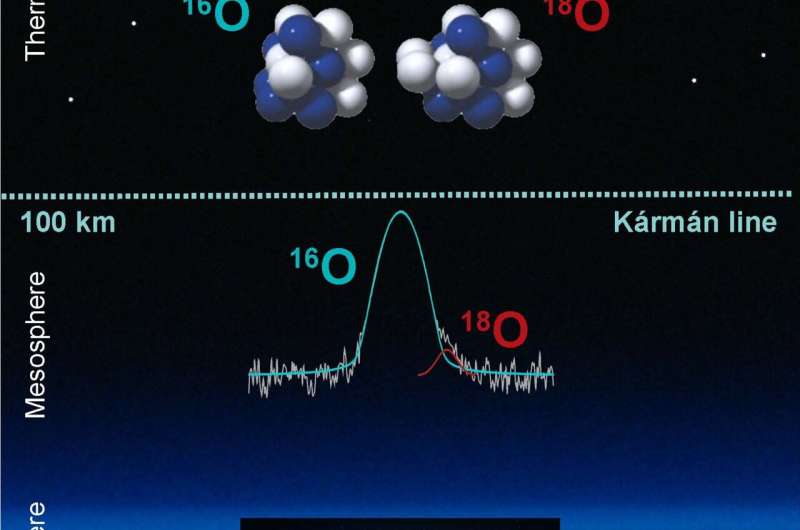
A study of the upper atmosphere's composition has successfully measured an increased presence of 18O, a heavier oxygen isotope with 10 instead of eight neutrons. Helmut Wiesemeyer (MPIfR Bonn) and his colleagues have measured the 18O fraction of the upper mesosphere/lower thermosphere for the first time, using the GREAT instrument aboard SOFIA and found that the upper atmosphere has an 18O fraction close to that of the lower atmosphere.
A better understanding to what extent biological effects permeate Earth's atmosphere could someday help researchers refine their search for potential signs of life on other planets.
Where is the boundary between Earth's atmosphere and space? A seemingly simple question, yet it has no straightforward answer. Aerospace applications refer to the so-called Kármán line, defined at an altitude of 100 km above sea level. It is an altitude at which hydrodynamic lift definitely ceases, or at which satellites cannot finish a single orbit around Earth, due to the friction with the air in the upper atmosphere.
On the other hand, only recently a magnetospheric wind has been discovered, traveling from Earth's ionosphere all the way to the moon, where it contaminates the isotopic composition of the soil, exposed to the solar wind.
This terrestrial fingerprint could be considered unique in the solar system because it potentially carries a signature of respiratory metabolism. The Dole effect describes an inequality in the ratio of the heavy isotope 18O to the lighter 16O, measured in the atmosphere and in seawater. The reason is as follows: oxygen, produced as a waste product of photosynthesis, mainly inherits its isotopic composition from that of the water supply, while respiration preferentially destroys the lighter version of oxygen.
Efficient vertical mixing carries this well-studied bio-signature up to the stratosphere. Further mixing of air into the higher atmospheric layers (the mesosphere and the thermosphere) has been evidenced a decade ago. The thermosphere is the basepoint for the wind of oxygen ions emerging into Earth's plasma sheet, yet its isotopic oxygen composition is still unknown.
"In our attempt to remotely measure the isotopic composition of oxygen in the mesosphere and lower thermosphere of Earth, we use a relativistic effect, thanks to which the electronic ground state of atomic oxygen splits into three fine-structure levels," says Helmut Wiesemeyer from the Max Planck Institute for Radio Astronomy MPIfR), the leading author of the publication.
"Radiative transitions from one quantum state to another generate infrared spectral lines. They are further split when one adds one or two neutrons to the nucleus: the atom's center of gravity is displaced, resulting in a slight change of the characteristic fine-structure line frequencies."
Originating in the mesosphere and lower thermosphere of Earth, these spectral lines appear in strong absorption against infrared-bright background sources, and therefore provide valuable fingerprints of the chemistry in this region.
"For the first time, we could identify the spectroscopic signature of this isotope shift in spectral lines of atomic oxygen in nature. It is in an environment far from earthbound laboratories, and difficult to access for in-situ studies—too high for balloons, and too low for Earth-orbiting satellites," explains Rolf Güsten, also from MPIfR, who was, till 2018, the principal investigator for the GREAT instrument onboard SOFIA which made the detection possible.
"Our observations allow to identify the spectral line of 18O in the Terahertz regime in absorption against the moon."
"Here we have come full circle: the strength of the spectral line from heavy 18O, with respect to its equivalent from the main isotope 16O, allows us to remotely measure the relative abundance of both species," adds Jürgen Stutzki from Cologne University, who took over as PI for the GREAT instrument in October 2018.
"Based on measurements from a stratospheric observatory, we deduce values that are representative of the lower atmosphere, rather than of the solar wind dominating where the interplanetary magnetic field takes over from that of Earth."
Yet the jury is still out: at the sensitivity achieved so far, it cannot be decided yet whether the biogenic isotope ratio characterizing molecular oxygen dominating the lower atmosphere or that of stratospheric ozone is traced. More measurements are needed to achieve a higher sensitivity. A rewarding endeavor, also because the origin of the isotopic record of ozone is not yet fully understood; it is thought to arise from a class of fast chemical reactions exchanging isotopes among their partners.
"We show that in the mesosphere and lower thermosphere these reactions compete with inelastic collisions exciting quantum states without changing either electric charge or chemical bonds. This competition entails a non-equilibrium population of the ground-level quantum states of 18O, not considered by previous studies, and in contrast to the thermodynamic equilibrium found in 16O," says Heinz-Wilhelm Hübers from the DLR Institute of Optical Sensor Systems in Berlin.
"The relative strengths of the measured spectroscopic lines are crucial for evidencing the different population distributions. Together with empirical data for the upper atmosphere's concentrations of atomic and molecular oxygen, this is sufficient to correct our determination of the isotopic fractionation. Our observations with the balloon experiment OSAS-B are going in that direction."
At first sight, the need for such a correction seems to add unwanted complexity to the analysis. At second glance, it provides a tool to study the impact of isotopic exchange reactions between atomic and molecular oxygen occurring before ozone forms by well-known chemistry. This requires a third body serving as a catalyst, abundant in the stratosphere but increasingly rare at the targeted higher altitudes in both, mesosphere and thermosphere.
Last, but not least, selection rules imposed by quantum theory imply a strong dependence of the speed of collisional excitation on temperature, competing with the exchange of isotopes. This effect might ultimately be used as a complement to corroborate empirical models of the upper atmosphere.
"At the time of writing these lines, we are not yet ready: future experiments will be needed to come to a final result, monitoring the infrared sky, in continuation of successful airborne observing programs," concludes Helmut Wiesemeyer.
The study is published in the journal Physical Review Research.
More information: Helmut Wiesemeyer et al, First detection of the atomic O18 isotope in the mesosphere and lower thermosphere of Earth, Physical Review Research (2023). DOI: 10.1103/PhysRevResearch.5.013072
Provided by Max Planck Society




















