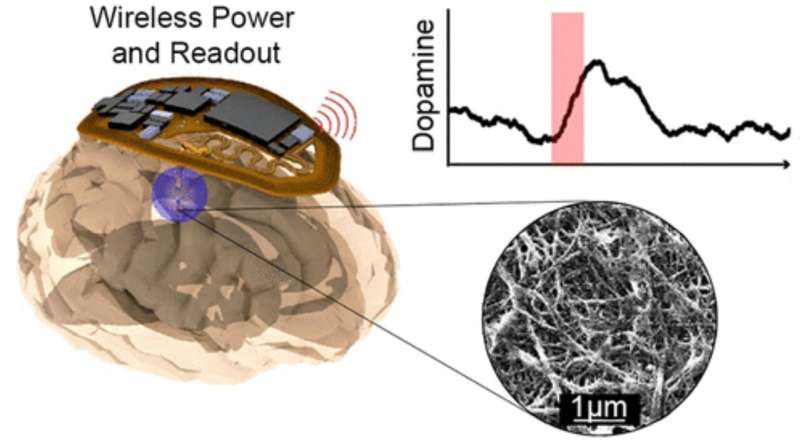
Graphicl abstract. Credit: ACS Nano (2022). DOI: 10.1021/acsnano.2c09475
Scientists have developed a wireless, battery-free implant capable of monitoring dopamine signals in the brain in real-time in small animal models, an advance that could aid in understanding the role neurochemicals play in neurological disorders.
The device, detailed in a study published in ACS Nano, activates or inhibits specific neurons in the brain using light, a technique known as optogenetic stimulation. It also records dopamine activity in freely behaving subjects without the need for bulky or prohibitive sensing equipment, said John Rogers, Ph.D., the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery, and a co-author of the study.
"This device allows neuroscientists to monitor and modulate brain activity in real-time and in a programmable fashion, in mice—a very important class of animal model for neuroscience studies," Rogers said.
"The device's miniature size, extremely lightweight construction and its wireless, battery-free mode of operation enables behavioral studies that would otherwise be impossible. We're able to watch how the brain is operating as an animal is moving and engaging in naturalistic environments in an unimpeded manner, in isolated individuals or in socially interacting groups."
To create the device, investigators first designed a miniaturized dopamine sensor using carbon-fiber composite electrodes to offer the highest sensitivity possible. Then, they implemented wireless, battery-free electronic circuits powered via external transmission antennae and fabricated the device using laser-patterning and thin film deposition to create a tiny, flexible implant.
The device is 50 times lighter and 10 times smaller than the most advanced alternative, according to the study authors.
In the study, the device was able to stimulate regions of the brain and record dopamine activity in response to opioid and naloxone exposures in mouse models.
The technology will be useful in studying how neurochemicals affect behavior, addiction and the development of neurological diseases, said Philipp Gutruf, Ph.D., assistant professor of Biomedical Engineering at the University of Arizona and senior author of the study.
"Neurochemical composition in the brain is an area that, because of the current limitation of research tools, is not as well understood as electrical activity," said Gutruf, a former postdoctoral fellow in the Rogers laboratory. "However, neurochemical composition is indicated to be very important in the treatment and detection of neurodegenerative diseases such as Alzheimer's and Parkinson's. Advances introduced by our work will help the neuroscience community to understand fundamental mechanisms and validate new therapy candidates."
Moving forward, the study authors hope to refine the device and make it commercially available, Rogers said.
"We want to extend the chemical sensing capabilities to other neurotransmitters besides dopamine and allow for it to interface with multiple regions of the brain simultaneously," said Rogers, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. "Additionally, we'd like to be able to make this technology available to the broader community of neuroscientists in a low-cost way to facilitate their own research efforts."
More information: Tucker Stuart et al, Wireless, Battery-Free Implants for Electrochemical Catecholamine Sensing and Optogenetic Stimulation, ACS Nano (2022). DOI: 10.1021/acsnano.2c09475
Journal information: ACS Nano
Provided by Northwestern University