Credit: Delft University of Technology
Spiropyrans are chemicals that change color when you shine light on them—a useful property in many areas of medicine and technology. Now 3mE's Georgy Filonenko (MSE) and his colleague, Richard Janissen at the Faculty of Applied Science have discovered a new use for these so-called photochromic molecules—visualizing stresses in solid polymers. Their research is published in the Journal of the American Chemical Society.
"Every time you make a polymer solution into a solid, it comes under stress," explains Filonenko, "and we have found a way to see those stresses inside the polymer."
Spiropyrans
It was known in the field that if you cool down a molten polymer, anomalies are created as it becomes solid so Filonenko and Janissen decided to try and check "a wild assumption" that small molecules can somehow "feel" when the polymer becomes solid.
"We needed to pick a molecule that changes color easily, that we can always look at and measure easily," says Filonenko. "It was endlessly naïve now that we think about it but we just picked one that's been known about for about 50 years—spiropyran—which can be colored or discolored by heat or light or mechanical stimuli."
Video recordings of photoswitching for p-CE. Credit: Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c11280
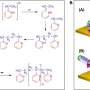
Spiropyrans are organic molecules that exist as isomers—one isomer is colored and the other is colorless. It turns out however that these isomers are also different lengths.
"We figured out that the colored molecule is a little bit longer than the colorless form of the molecule and if your reaction is associated with a change in the length, then if your polymer is somehow under mechanical stress, then the reactions that shrink the molecular size will be promoted by compression, and reactions that elongate the molecules will be promoted by tension. When we realized that, our data started making sense," say Filonenko and Janissen.
What Filonenko and Janissen saw was that when the polymer became solid, there are huge changes in the reaction rate, the so-called kinetics of the reaction. "We saw such huge changes in the kinetics of the transformation between the different forms of the molecule, the colored and the colorless, we realized that this had to be something major and that, we figured out, was the internal stresses within the polymer formed as the polymer solidified."
More information: Richard Janissen et al, Mechanochemistry of Spiropyran under Internal Stresses of a Glassy Polymer, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c11280
Journal information: Journal of the American Chemical Society
Provided by Delft University of Technology