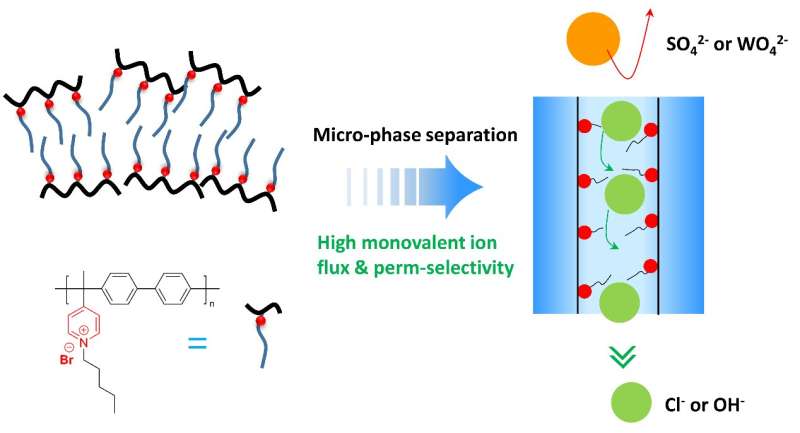
Poly(alkyl-biphenyl pyridinium) AEMs with a hydrophobic side chain were fabricated, showing high anion perm-selectivity in NaCl/Na2SO4 and NaOH/Na2WO4 systems. Credit: Tongwen Xu, University of Science and Technology of China
Monovalent anion perm-selective membranes (MAPMs) combined with electrodialysis can simultaneously realize the efficient separation of mono-/di-valent anions and the concentration of monovalent salt. However, their applications in practical industrial scenarios are limited due to the low anion selectivity of commercial MAPMs, especially the poor alkali stability.
Recently, a team of scientists has constructed poly(alkyl-biphenyl pyridinium)-based anion exchange membranes with hydrophobic side chains that provide excellent permselectivity to separate monovalent ions such as chloride (Cl−) and hydroxide (OH−) from other multivalent ions in electrodialysis. Their work is published in the journal Industrial Chemistry & Materials (ICM) on January 2023.
Monovalent ion perm-selective membranes can achieve precise ion separation, which has important needs in various fields such as lithium extraction from salt lakes, brine refining, and zero discharge of high-salt wastewater. As for the MAPMs, the biggest challenges are the low anion selectivity and the poor alkali resistance.
"The selective separation of mono-/di-valent anions involves the recycling of high salt wastewater, the purification of brine in chlor-alkali industry, and the recovery of waste alkali. How to construct MAPMs with high anion selectivity and high alkali stability is the direction of our team's efforts," explains Tongwen Xu, a professor at the University of Science and Technology of China.
Anion exchange membranes (AEMs) are usually fabricated by bromo- or chloromethylation of polymers and then quaternization via a Menshutkin reaction. However, this method needs highly toxic chemicals such as chloromethylation or bromination reagents that may risk the environment. The ionic conductivity and alkaline stability of such membranes are often low because the main chain's cationic head groups have low mobility; can barely undergo a good microphase separation to create efficient channels for OH− ion conduction.
In addition, benzyl groups, which commonly exist in the primary chain AEMs, are quickly attacked by the OH− ions and encounter nucleophilic substitution reactions. Higher ion exchange capacity is advantageous for OH− transportation but may increase water uptake. The AEMs may swell severely, lowering the separation efficiency, and exacerbating the attack of OH− on the membranes, thus damaging their chemical and mechanical robustness.
The researchers offered a chemically robust polymer backbone with a more elongated side chain group to overwhelm the above-mentioned problems in the traditional AEMs. In the resulting MAPMs, the microphase separation appears between long-side chains and a hydrophobic polymer.
This is equivalent to building a selective anion transport channel in the membrane, which is favorable to the active transport of Cl− compared with SO42− ion giving a high permselectivity. It also showed a high OH− flux with a high permselectivity between OH−/WO42−, much higher than the commercial Neosepta ACS membrane.
Based on the design of an ether-bond-free polymer backbone, the MAPMs also showed excellent alkali stability after twenty runs.
"We also found that these novel MAPMs with significant microphase separation morphology can separate bromide, fluoride, and nitrate ions from other multivalent anions based on the differences in hydration radius and Gibbs hydration energy with high selectivity," said Xu.
The researchers also revealed that they have completed a pilot-scale preparation of the MAPMs. The comprehensive evaluation of the performance of the pilot-scale MAPMs is still in progress. They plan to commercialize the MAPMs at their earliest to solve some of the industry's difficulties in the anion separation.
More information: Hongxin Yang et al, Poly(alkyl-biphenyl pyridinium) anion exchange membranes with a hydrophobic side chain for mono-/divalent anion separation, Industrial Chemistry & Materials (2023). DOI: 10.1039/D2IM00043A
Provided by Institute of Process Engineering, Chinese Academy of Sciences