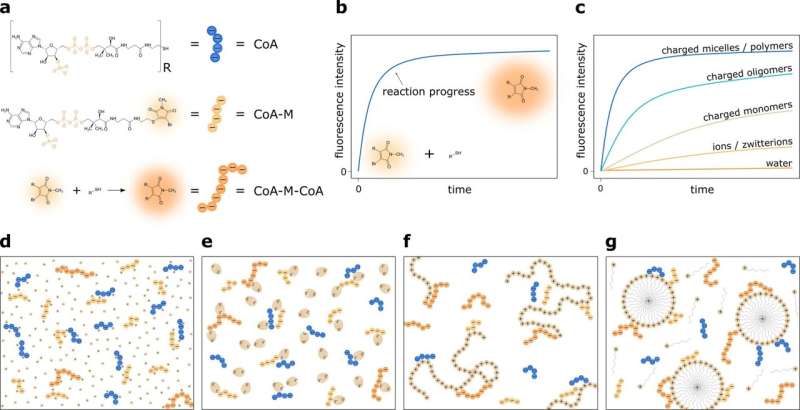
Schematic representation of the experimental system. a The reaction between negatively charged coenzymes A (CoA) is used as a model reaction. One of them—CoA-M—has fluorescent moiety. The reaction between reactants occurs through the substitution of bromine in the CoA-M by the thiol group of CoA. This process is practically irreversible, and as a product, CoA-M-CoA molecule is formed. b The product formation is accompanied by an increase in fluorescence intensity. c Here, we show how the charge screening and enhancement of local concentration of reactants at the oppositely charged molecules' surfaces increase reaction rates of Coenzyme A molecules. We investigate the reaction speed up in the aqueous solution in the presence of d ions and charged monomers, e zwitterions, f charged oligomers and polymers, and g charged micelles. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-34182-z
The occurrence of chemical reactions between like-charged compounds in aqueous solutions is very slow since particles repel each other. A recent breakthrough published in Nature Communications shows a new way to control chemical reactions by charge neutralization and increase in effective concentrations of reactants. The joint research teams led by prof. Robert Hołyst from the Institute of Physical Chemistry, Polish Academy of Sciences, discovered that using compounds with a large surface charge density speed up the reactions up to 5-million fold.
The synthesis of new materials or manufacturing materials is very complex on the molecular level. Let's consider two molecules that have like charges. Due to the repulsion, they remain far from each other and seldom come close enough for the reaction to happen. This kind of reaction in pure water can take days or even weeks to complete, but its rate can be drastically shortened with a bit of spark. Such a spark comes from natural catalysts, like enzymes or synthetic nanozymes that mimic natural ones as well as metal-based catalysts widely used in industry. No matter their origins, they boost the tempo of reactions. However, they are usually selective for specific reactions.
Recently, a joint research team from the Institute of Physical Chemistry, Polish Academy of Sciences, University of Zurich, Radboud University, Lancaster University, University of Oxford, and the University of Warsaw led by prof. Robert Hołyst demonstrated that the acceleration of the reaction rates between two like-charged biologically active molecules depends on the nature and form of the charge in the aqueous solution.
As the model reaction, the complex formation between two negatively charged compounds—Coenzyme A molecule and its derivative repulsing each other—was investigated. This process is much slower in aqueous media compared to organic solvents. Therefore, it was exposed to the counter-charged compounds to boost the reaction rate in water.
The scientists checked molecules of different sizes and charge quantities like ions, monomers, zwitterions, oligomers, polymers, and even micelles. The results were remarkable. The reaction rate constant was increased 5 million times by the positive charge micelles. In contrast, monomers, oligomers, and polymers enhanced the reaction rate 103–105-fold, while ions or zwitterions increased the tempo of reaction only ~1000 folds (i.e., conventional screening).
Adam Kowalski remarks, "In this work, we aimed to answer whether the reaction rate enhancement by 'counter-charged' species could be a general phenomenon and explained the theoretical basis of such electrostatic catalysis. Our paper shows that reaction rates can be controlled within several orders of magnitude by tuning the magnitude and spatial distribution of the electric charge of the catalyst."
How does it work? The exposition of Coenzyme A (CoA) or methylmaleimide substituted Coenzyme A (CoA-M) on the compounds having opposite charges disturbs the repulsion between negatively charged CoA and CoA-M. When the size of added compounds is the same or larger than the Debye length, negatively charged molecules with specific concentrations, neglect the repulsion in positively charged agents, getting closer to each other.
Hence, the added compound screen the molecules' repulsion forces, making this process a specific case of catalysis. Researchers also tested ion-sensitive DNA hybridization to determine whether described effect works for more complex processes. Similarly to the CoA and CoA-M reaction, positively charged compounds speed up the reaction rate, confirming the electrostatic nature of catalysis.
"We accelerate the chemical reaction in water by 5 million times using an unusual but simple approach, positively charged surfactants (so practically a soap). This phenomenon was demonstrated in a covalent reaction between two coenzymes A and non-covalent complex formation—DNA duplex. We are encouraging chemists and biochemists to speed up other prolonged reactions between like-charged compounds they observed. In addition, our findings can open the pathway for carrying out certain reactions in water with surfactants that by now were performed in organic solvents," claims Dr. Grzegorz Bubak.
As the micelles of cetrimonium chloride (CTAC), benzethonium chloride (BTC), and cetylpyridinium chloride (CPC) are much larger than the other multi-charged compounds and surfactants, their surface charge makes them a "contact point" for the other molecules. This effect is observed in chemical reactions and dimerization of DNA, including the interaction of two negatively charged molecules.
So, this unique catalytic effect can also be achieved for physical processes enhancing molecules' transport. The experimental results presented by researchers prove that the rise of the reaction rate correlates directly to the catalyst's surface charge in the system. Hence, controlling the compound's charges enables controlling the kinetics of specific processes.
Dr. Paweł Zuk says, "The experimental results, guided a predictive theoretical model. The catalytic mechanism is the increase in the rate of substrate encounters. We are confident that it is applicable to other particles too."
This breakthrough brings us closer to future sensing applications to detect specific ions in very diluted solutions or interactions between particular molecules. Researchers also highlight the importance of interdisciplinary collaboration. Their joint findings open the door to control chemical reactions even on a tiny scale.
More information: Adam Kowalski et al, Effective screening of Coulomb repulsions in water accelerates reactions of like-charged compounds by orders of magnitude, Nature Communications (2022). DOI: 10.1038/s41467-022-34182-z
Journal information: Nature Communications
Provided by Polish Academy of Sciences