This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A mechanically interlocked molecule that can be controlled by light
Catalysts boost many types of chemical reactions, from our bodies to the industrial production of compounds and controlled fuel combustion in a car. From solid to gaseous, no matter their formula, their role is to enhance the rate of chemical reactions to make many processes easier.
But what if some molecule could work as both the inhibitor and catalyst in a controlled way? Many processes would be much easier to control. Tackling this issue, researchers from the Institute of Physical Chemistry, Polish Academy of Science, led by Prof. Sashuk proposed a fascinating mechanically interlocked molecule that can be controlled by light.
Supramolecular chemistry delivers many complex molecules assembled by noncovalent, intermolecular bonds and bottom-up nanofabrication. This branch of chemistry deals with the processes inspired by nature, bringing unique complex structures with completely different properties than the single components. But how does it work? It all starts with the type of the molecules. Some reactions can be inhibited or catalyzed depending on the chemical properties of assembled molecules.
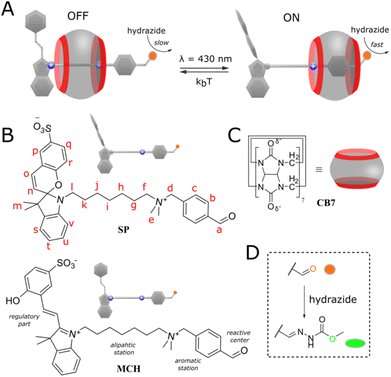
Prof. Sashuk and his team have presented a mechanically interlocked molecular architecture that can be controlled with light working as a photo-switchable set of molecules that selectively regulate the rate of particular chemical reactions. Specifically, they have focused on the design, synthesis, and application of a molecule that can control the position of another molecule on its axis. In that way, the positioned molecule could be closer or further from the reaction center installed on the same axis.
To create this they have proposed a semirotaxane, a complex of molecules in which a rod-shaped molecule is threaded through and partially trapped in a hoop-shaped molecule called a macrocycle. The rod-shaped molecule contains two stations, where one, benzaldehyde, works as the site where the reactions occur, and the second one—a photo-switch terminated heptyl is the reaction regulator. Both stations are separated by a dimethylammonium group keeping the macrocycle, namely cucurbit[7]uril, on the axis by the coulombic stabilization.
Prof. Sashuk remarks, "We developed a new type of regulation of supramolecular catalysis. Photo-switchable inhibitor linked with substrate into one molecule impedes the increase in the reaction rate upon increasing the amount of catalyst. After deactivation of the inhibitor with light, the system starts to exhibit the typical catalysis enhancement until the saturation of the reaction site. Importantly, the prepared semirotaxane can regulate not only self-reaction but also the outcome of external reactions."
Under the application of light in the blue region, researchers observed the acceleration of the C−N coupling reaction called hydrazonation. So far, the macrocycle working as a catalyst that usually preferred to stay at the heptyl station, due to the weakening of electrostatic interactions, changes its position coming closer to the second benzaldehyde station promoting the reaction with incoming hydrazide. The researchers detected the rate of the hydrazonation reaction being about 5.4 times higher than the rate observed in the dark.
"Importantly, the acceleration of the reaction can be done at any time. Furthermore, after the reaction is complete, the catalytic system can be readily reinstated by lowering the pH of the solution," claims Dr. Nazar Rad.
Interestingly, when two types of hydrazides are present in the reaction mixture, the rod-shaped molecule can selectively react with one of them and change the final product ratio. The researchers explain this phenomenon by the different affinity of the macrocycle to the products formed.
This work is a step forward in developing new types of regulation in catalytic systems with remote control. Currently, the team is working on adapting the presented system for various purposes, including complex chemical processes where the reaction requires selectivity.
More information: Nazar Rad et al, A light-gated regulation of the reaction site by a cucurbit[7]uril macrocycle, Chemical Science (2022). DOI: 10.1039/D2SC02077G
Journal information: Chemical Science
Provided by Polish Academy of Sciences