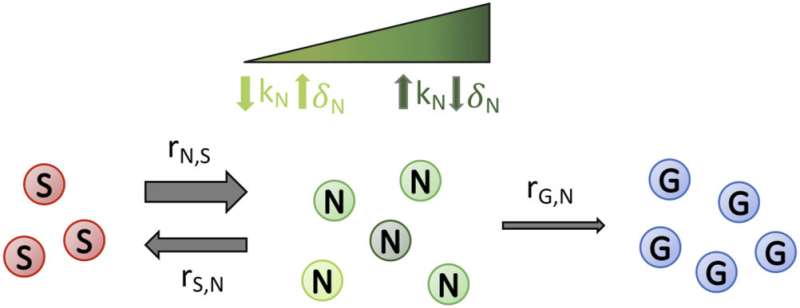
Schematic depicting the transitions between susceptible, non-genetically resistant, and genetically resistant subpopulations in a cell population undergoing drug treatment. Cells with the drug susceptible (S) phenotype can switch to non-genetically drug-resistant (N) phenotype and vice versa (at rates rN,S and rS,N, respectively). The degree of susceptibility or resistance of S, N, and G to a drug can be varied between simulations and is dependent on the treatment scenario being investigated. For example, a higher birth rate (kN) or a lower death rate (δN) results in the degree of transient drug resistance of N cells being higher (represented by dark green cells), whereas a lower kN or a higher δN results in a lower level of transient drug resistance (represented by light green cells), as described overall by the fitness (N subpopulation growth rate) in the presence of a static or cidal drug, respectively; this is similar, but not shown in the schematic for clarity, for S and G cells. Cells from the N subpopulation can mutate (at a rate rG,N) to become permanently genetically drug-resistant (G) cells. Credit: Physical Biology (2022). DOI: 10.1088/1478-3975/ac8c17
Researchers are investigating how disease-causing fungi become resistant to antifungal drugs to help prevent potentially devastating consequences of the growing resistance harmful microbes are developing to drugs.
"Antimicrobial drugs are fundamental for us to live healthy lives," says Daniel Charlebois, assistant professor in the Department of Physics, adjunct professor in the the Department of Biological Sciences and co-author of a recent study published in Physical Biology.
When microbes infecting a person's body form permanent resistance to a drug, that drug becomes ineffective or less effective in battling the infection it's designed to target.
Cells develop resistance to antimicrobial drugs in two ways, Charlebois explains: genetically or non-genetically. Genetic resistance is permanent and passed down to offspring. It's only in the past decade or so that researchers have begun digging into non-genetic resistance. A missing piece of the puzzle is what happens when cells are transitioning between these two resistant states—and that's what Charlebois hopes to shed light on with his work.
Understanding the transition between the two types of resistance is important to find ways to prevent cells from mutating and evolving to develop genetic resistance.
"People tend to be experts in one or the other forms of resistance, and it's only now that we're really starting to look at this interplay," says Charlebois.
"Trying to understand how these non-genetic mechanisms influence evolution is really interesting for drug resistance, which is an evolutionary process."
Charlebois' study showed that when both genetically and non-genetically resistant cells are present, it takes longer for the population of cells to become fully genetically resistant because the two types of cells are competing for resources such as nutrients and space.
"It takes longer for those genetically resistant mutants to spread and become more than 95% of the population," says Charlebois.
Once a cell mutates to become genetically resistant, it remains that way. However, a non-genetically resistant cell can become a "susceptible cell," meaning it will die or stop growing in the presence of the drug. With the resistance removed, an infection can be treated.
To examine the different subpopulations of resistant cells, Charlebois and physics master's student Joshua Guthrie created a population model. "We are considering the population dynamics, whereas before it was just considered one population [of resistant cells]," explains Charlebois.
Guthrie developed code and did the simulation work to streamline the process and narrow down the number of experiments that would need to be done in the lab. The modeling portion of the research provided key information, such as when the first mutation happens in a cell.
"That might inform when you should add or change drugs during treatment," says Charlebois.
More knowledge about the interplay between the different resistance mechanisms could give physicians critical information about what type of drugs to administer and what treatment schedule would be most effective.
For example, alternating drugs to exploit trade-offs in susceptible, non-genetically resistant, and resistant subpopulations during treatment may help keep the genetically resistant subpopulation at bay for longer. This could inform treatment options such as sequential or combination therapies, says Charlebois.
"You're dealing with a population of interacting entities, different types of potential drug resistance," says Charlebois. "Thinking of ways to exploit that is not something that is typically considered when you're developing treatments."
More information: Joshua D Guthrie et al, Non-genetic resistance facilitates survival while hindering the evolution of drug resistance due to intraspecific competition, Physical Biology (2022). DOI: 10.1088/1478-3975/ac8c17
Journal information: Physical Biology
Provided by University of Alberta