This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cubes outperform spheres as catalyst particles
To date, nanoparticles as catalysts for green hydrogen have been like rowers in an eight: researchers could only measure their average performance, but couldn't determine which one was the best. This has now changed following the development of a new method by the group led by Professor Kristina Tschulik, head of the Chair of Electrochemistry and Nanoscale Materials at Ruhr University Bochum, Germany.
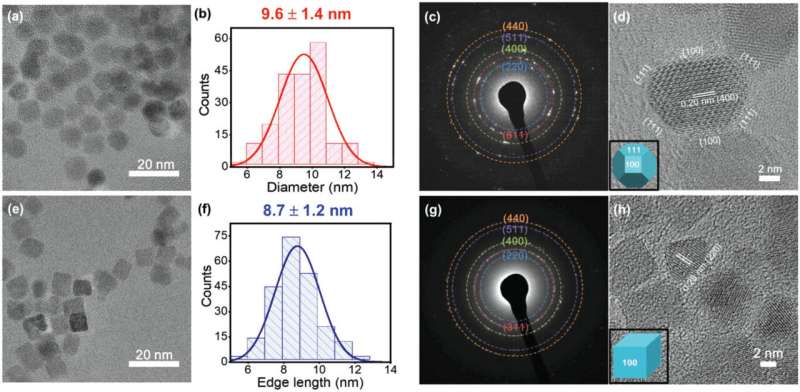
In collaboration with researchers from the University of Duisburg-Essen, she successfully proved that cube-shaped cobalt oxide nanoparticles are more efficient than spherical ones. This paves the way for the systematic design of cost-effective and efficient catalysts for green hydrogen. The researchers reported their findings in the journal Advanced Functional Materials on January 3, 2023.
How to make electrolysis competitive
The world must reduce CO2 emissions in order to combat climate change. To this end, so-called gray hydrogen is widely used today, which is obtained from oil and natural gas, but efforts are made to replace it with green hydrogen, which comes from renewable sources. Green hydrogen can be produced by electrolysis, a process where electricity is used to split water into hydrogen and oxygen. However, several challenges still need to be tackled to render electrolysis a competitive approach.
At present, the water splitting process is only efficient to a limited degree, and there are not enough powerful, durable and cost-effective catalysts for it. "Currently, the most active electrocatalysts are based on the rare and expensive precious metals iridium, ruthenium and platinum," lists Tschulik. "As researchers, our job is therefore to develop new, highly active electrocatalysts that are free of precious metals."
Her research group studies catalysts in the form of base metal oxide nanoparticles that are a million times smaller than a human hair. Manufactured on an industrial scale, they vary in shape, size and chemical composition. "We use measurements to examine so-called catalyst inks, in which billions of particles are mixed with binders and additives," outlines Tschulik.
This method only allows researchers to measure an average performance, but not the activity of individual particles—which is what really matters. "If we knew which particle shape or crystal facet—the surfaces that point outwards—is most active, we could specifically produce particles with that exact shape," says Dr. Hatem Amin, postdoctoral researcher in analytical chemistry at Ruhr University Bochum.
Winner of the nanoparticle race
The research group has developed a method to analyze individual particles directly in solution. This enables them to compare the activity of different nanomaterials with each other in order to understand the influence of particle properties such as their shape and composition on water splitting.
"Our results indicate that cobalt oxide particles in the form of individual cubes are more active than spheres, as the latter always have several other, less active facets," say the researchers.
Theory confirms experiment
The Bochum group's experimental findings were confirmed by its cooperation partners headed by Professor Rossitza Pentcheva from the University of Duisburg-Essen as part of the Collaborative Research Center/Transregio 247. The latter's theoretical analyses indicate a change in the active catalyst regions, namely from cobalt atoms that are surrounded by oxygen atoms forming an octahedron to cobalt atoms that are surrounded by a tetrahedron.
"Our insights into the correlation between particle shape and activity lay the foundation for knowledge-based design of viable catalyst materials and, consequently, for the transformation of our fossil energy and chemical industries toward a circular economy based on renewable energy sources and highly active, long-lasting catalysts," concludes Tschulik.
More information: Zhibin Liu et al, Facet‐Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction, Advanced Functional Materials (2022). DOI: 10.1002/adfm.202210945
Journal information: Advanced Functional Materials
Provided by Ruhr-Universitaet-Bochum





















