This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Critical impacts of interfacial water on C-H activation in photocatalytic methane conversion
Non-thermal activation and utilization of methane, the main component of natural gas and a ubiquitous natural carbon resource, are among the global challenges for achieving sustainable society. However, incomplete knowledge on microscopic mechanisms of methane activation and hydrogen formation hampers the development of engineering strategies for the reaction system.
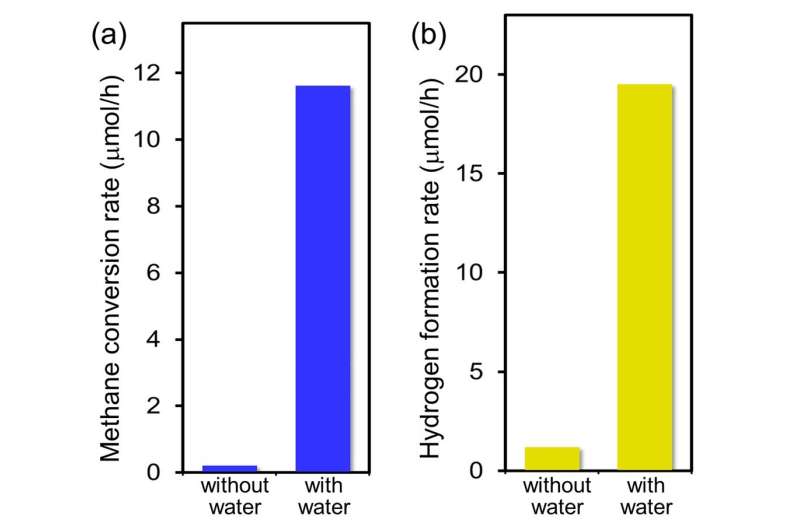
Very recently, in a study published in Communications Chemistry, researchers led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, succeeded in obtaining key molecular-level insights into the crucial role of interfacial water on the non-thermal C-H activation in photocatalytic methane conversion. Combining real-time mass spectrometry and operando infrared absorption spectroscopy with ab initio molecular dynamics simulations, they showed that methane conversion is hardly induced by the direct interaction with the trapped hole at the surface Olat site; instead, activation is significantly promoted by low barrier hydrogen abstraction from methane by photoactivated interfacial water species.
In water-mediated processes, the photocatalytic C-H activation is not the rate-determining step, which is in stark contrast to the case of traditional thermocatalytic methane reforming. Moreover, owing to the moderate stabilization of •CH3 in the hydrogen-bond network of water, overall photocatalytic conversion rates are dramatically improved by typically more than 30 times at ambient temperatures (~300 K) and pressures (~1 atm). As essentially opposed to thermal catalysis, methane photocatalysis no longer requires high-pressure methane gas (> 20 atm) in the presence of adsorbed water layer.
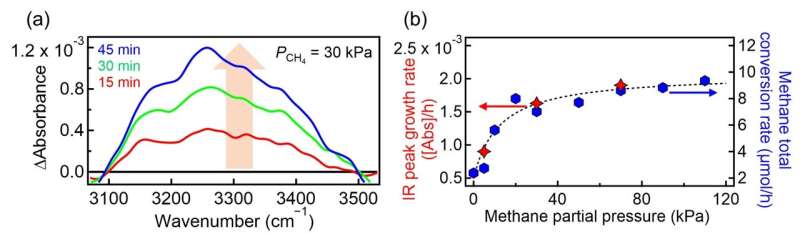
Water-assisted effects are noticeable also in ethane formation, although water is not explicitly involved in the homocoupling reaction equation (2CH4 → C2H6 + H2). These results indicate that interfacial water kinetically plays crucial roles beyond the traditional thermodynamic concept of redox potential, in which oxidation of water by surface trapped holes is less thermodynamically favored than methane oxidation: E°•OH/H2O = 2.73 V and E°•CH3/CH4 = 2.06 V versus the standard hydrogen electrode.
Notably, these water-assisted effects are commonly observed for several representative photocatalysts with different band-gap energy, such as TiO2, Ga2O3, and NaTaO3, indicating that the incorporation of methane into photoactivated interfacial hydrogen-bond network is essential key for the non-thermal activation of methane.
This work not only expands the molecular-level understanding of the non-thermal C-H activation and conversion, but also provides a fundamental basis for the rational interface design of non-thermal catalytic systems toward the effective and sustainable utilization of methane under ambient conditions.
More information: Critical impacts of interfacial water on C–H activation in photocatalytic methane conversion, Communications Chemistry (2023). DOI: 10.1038/s42004-022-00803-3
Provided by National Institutes of Natural Sciences