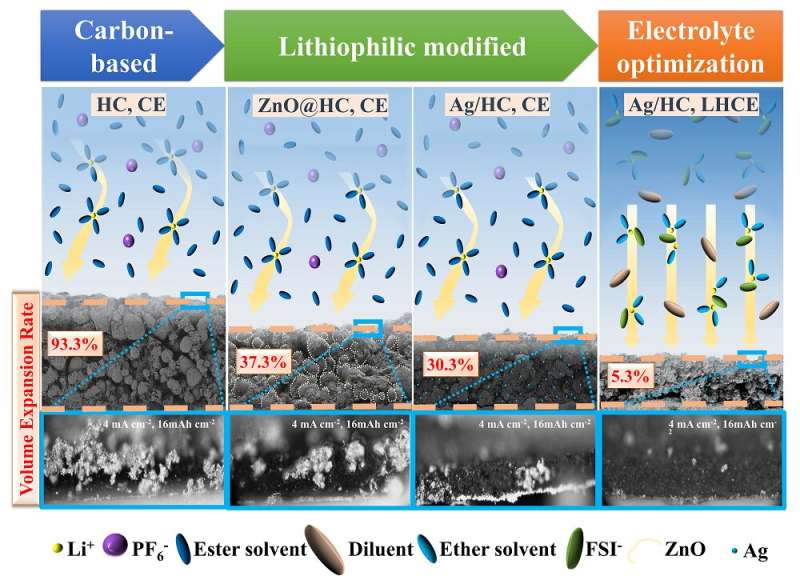
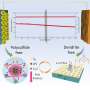
A schematic illustration of the synergistic improvement of lithium deposition behavior in hydrocarbon host structures by lithiophilic sites and localized high-concentration electrolytes, which shows three significant reductions in both electrode thickness and dendrites growth. Credit: Nano Research, Tsinghua University Press
While lithium metal is an ideal anode for next-generation high-energy-density batteries, there are challenges to be addressed before it can reach its full potential. A research team has conducted a study of lithium deposition behavior and developed a strategy involving hard carbon hosts that successfully addresses some of the problems currently facing the development of the lithium metal anode.
"This strategy addresses the volume change and dendrite problems by rationally designed host and electrolyte, providing a broad perspective for realizing lithium-metal anode," said Liping Wang, a professor at the University of Electronic Science and Technology of China.
The team published their research in the journal Nano Research.
Lithium metal is recognized as a promising anode for next-generation batteries because of its ultra-high theoretical capacity, extremely low electrode potential, and low density. However, lithium metal suffers from uncontrollable lithium dendrite growth, side reactions, and infinite relative volume change. These issues can lower the batteries' efficiency and shorten the battery cycle life, even possibly leading to short circuit or security risks.
Over time, researchers have suggested various strategies to alleviate dendrite growth and volume expansion. These strategies include constructing three-dimensional composite lithium anodes, optimizing the composition of electrolytes, applying artificial interphases, and using solid-state electrolytes.
The three-dimensional host is the most promising strategy to address the volume expansion and dendrite growth issues. Carbon-based materials are ideal host candidates for lithium-metal anodes because they are lightweight, have high conductivity and many pore structures, along with stable electrochemical/chemical properties.
"Yet even with these advantages, the challenges of volume expansion and dendrite growth have not been completely solved by the carbon-based host," said Wang.
More recently, researchers have explored modifying carbon materials with lithiophilic species (such as zinc, zinc oxide, aluminum, tin, silicon, silver, and magnesium) and developing suitable electrolytes as effective methods to improve the performance of these three-dimensional host materials.
"Yet the lithium deposition behavior and its intrinsic mechanism in these processes has not been systematically analyzed," said Wang.
To better understand the structure-activity relationship and guide the development of high-performance carbon-based host electrodes, the lithium deposition behavior and its intrinsic mechanism, the research team undertook their in-depth study. They used an optical microscope and a scanning electron microscope to systematically study the lithium deposition behavior of the hydrocarbon electrode under different surface modifications and electrolytes.
They found that lithium will not spontaneously deposit into the carbon pores, which is significantly dependent on the carbon surface, current density, areal capacity, and electrolyte.
So the team developed a lithiophilic modified commercial hard carbon with silver as a stable host. They discovered that the introduction of lithiophilic sites induced moderate dendrite growth and inhibited volume expansion.
They also discovered that localized high-concentration electrolytes proved to be more compatible with lithium and could optimize the lithium deposition morphology instead of the dendrite. Therefore, the silver/hydrocarbon electrode in the localized high-concentration electrolyte exhibited low volume change during cycling, achieved uniform and dendrite-free morphology of lithium deposition, and showed good long-term cycling with high efficiency over 316 cycles.
The team summarized their findings, explaining that although porous carbon has space to theoretically hold lithium, the lithium ions will not deposit into the expected pores because lithium atoms prefer to accumulate in explosive growth mode and are strong enough to prop up the carbon particles. They also discovered that surface modification on carbon can partially moderate lithium deposition with a decreasing nucleation barrier.
However, it is not significantly efficient as it is lithium-lithium deposition behavior after lithium is deposited on lithiophilic carbon. The team learned that using localized high-concentration electrolytes is more efficient in achieving a dendrite-free lithium deposition.
More information: Ge Zhou et al, Lithium deposition behavior in hard carbon hosts: Optical microscopy and scanning electron microscopy study, Nano Research (2022). DOI: 10.1007/s12274-022-5256-8
Journal information: Nano Research
Provided by Tsinghua University Press