Credit: CC0 Public Domain
Bacteria are small but tough organisms, partly because their cells are enclosed by a protective cell wall skeleton. Professor Felipe Cava and his team at Umeå University in Sweden and collaborators at Harvard Medical School in the U.S., have discovered long-sought proteins needed to maintain the bacterial cell wall structure. These proteins represent a very promising vulnerability for many bacteria that can be exploited by future antimicrobial compounds. The findings are published in Nature.
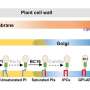
The cell wall, like the skin of animals, is essential for bacteria to stay alive. Many of our best antibiotics therefore target the proteins that build and remodel this structure. As the cell wall is located on the outside of the cell membrane that encloses the cell, its building blocks must be transported across this membrane from where they are made, the cytoplasm.
To carry out this transfer, bacteria use a specialized lipid carrier called undecaprenyl phosphate. Once these building blocks are delivered and assembled the lipid carrier must return to the cytoplasm to transport new units; however, the identity of the proteins recycling these lipids remained elusive until now.
Using the pathogen model organisms Vibrio cholerae and Staphylococcus aureus, the research team discovered that two poorly characterized protein families (DUF368 and DedA), which are widely conserved in all three kingdoms of life, are responsible for the recycling of the lipid carriers, the undecaprenyl phosphate lipids.
Interestingly, some of these proteins are only required under specific conditions suggesting that transporter usage is dynamic and regulated by diverse environmental cues. Importantly, lipid carrier recycling is vital for V. cholerae pathogenesis, suggesting that selective targeting of these transporters could be a viable approach for the development of novel antimicrobials.
"Bacteria normally experience a wide range of environmental changes both under free-living conditions and during infection. Selection of specific undecaprenyl phosphate transporter proteins to maintain cell wall stability in each environment might be an unexplored adaptative mechanism in bacteria," explains Dr. Emilio Bueno, Postdoctoral Researcher at the Department of Molecular Biology at Umeå university.
Motivated by an in vivo screen for V. cholerae intestinal colonization determinants, the team identified a multi-pass membrane protein which contains the widely conserved domain of unknown function, DUF368. Both V. cholerae and S. aureus, when lacking their respective DUF368-containing proteins, grew poorly and showed morphological defects that strongly implicated these membrane proteins in cell wall biogenesis, and particularly in the transport of undecaprenyl phosphate lipids.
"As our phenotypic data suggested that the mutants were defective in undecaprenyl phosphate re-internalization, we used a method that permitted us to quantify distinct lipid carrier species in membrane extracts," says Dr. Emilio Bueno.
Remarkably, although lipid carrier recycling is thought to be an essential function, DUF368 mutants were mostly affected at alkaline pH, thus suggesting the existence of other transporters for neutral and acidic pHs. A screen for synthetic lethal interactions identified a DedA family protein as an additional translocase of undecaprenyl phosphate.
The conditionality of distinct translocases could support lipid carrier flux in alternative microbial niches, for example, inside and outside the host. Together, these findings fill a major gap in the recycling pathway of undecaprenyl phosphate in bacteria and establish contexts that govern the activity of this critical function.
Undecaprenyl phosphate recycling is a key step in the biosynthesis of not only peptidoglycan, the primary structural component of the cell wall, but also other cell surface glycopolymers, including wall teichoic acids, certain lipopolysaccharide modifications, and capsules.
"Therefore, given its wide-ranging and critical role in cell surface maintenance, this step is an ideal target for antimicrobial therapies. Moreover, although DUF368 proteins are restricted to bacteria and archaea, DedA family members are widely present in eukaryotes, including humans. Thus, our findings may impact understanding of polyprenyl phosphate translocation across the kingdoms of life," says Felipe Cava, Professor at the Department of Molecular Biology at Umeå university.
More information: Brandon Sit et al, Undecaprenyl phosphate translocases confer conditional microbial fitness, Nature (2022). DOI: 10.1038/s41586-022-05569-1
Journal information: Nature
Provided by Umea University