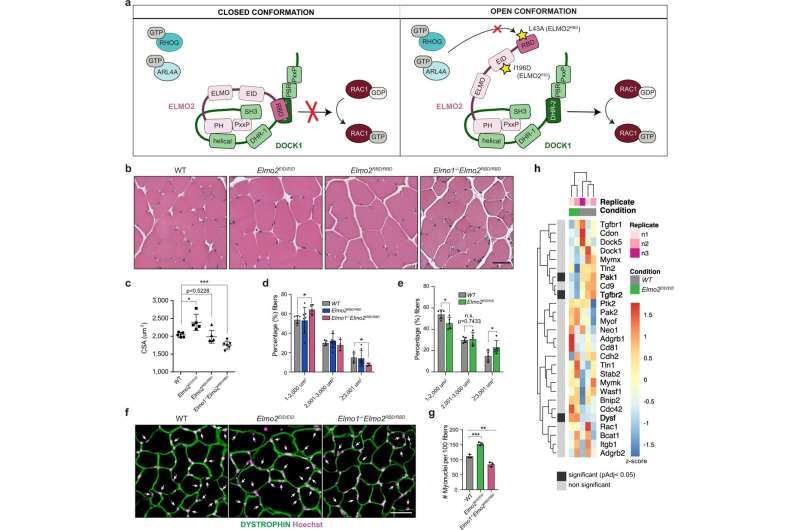
Manipulating ELMO2 conformational regulation impacts on myoblast fusion during muscle development and growth. a Schematic representation showing the closed and open conformations of the ELMO-DOCK complex. In the closed state, ELMO is in a closed conformation and the DHR-2 domain of DOCK is blocked by the RBD domain of ELMO, thus preventing RAC1 activation and the binding of interactors to the RBD of ELMO. Upon activation of the ELMO-DOCK complex, binding sites for ELMO interactors become available and the DHR2 of DOCK can activate RAC1. The RBD L43A mutation abrogates the binding of the RHOG and ARL4a GTPases, hence diminishing the signaling from ELMO/DOCK. The EID I196D mutation favors the open conformation of ELMO, which increases RAC1 activation by ELMO/DOCK. b Representative muscle fibers cross-sections of the indicated mice stained with H&E. c Quantification of b showing the mean myofibers cross-sectional area (CSA) +/−SD per genotype (n = 5 mice). d, e Distribution of myofibers size from (b) of the indicated mice. Data are presented as the mean values +/− SD (n = 5 mice). f Cross-sectional muscle sections of WT, Elmo2EID/EID and Elmo1−/−Elmo2RBD/RBD mice stained with anti-DYSTROPHIN (green) and Hoechst (magenta). Arrowheads indicate myonuclei located inside the myofibers. g Quantification of the number of myonuclei located inside the myofibers of e. Data are presented as the mean values +/− SD (n = 3 mice). (Scale bar: 50 µm). The Student’s t-test (for comparison of two independent groups) was used to calculate the P-values (two-tailed) presented in c–e, g; *P < 0.05, **P < 0.01, ***P < 0,001. h Expression heatmap of known fusogenes between WT and Elmo2EID/EID myoblasts. Samples and genes were clustered using Euclidian distance. P-values for differential expression were calculated using the Wald test and adjusted for multiple comparisons using the Benjamini-Hochberg method. Significantly differentially expressed genes (padj < 0.05) are represented by black boxes. Source data are provided as a Source Data file. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-34806-4
Neuromuscular disorders affect millions of people worldwide. Now a discovery made at the Montreal Clinical Research Institute of Montreal (IRCM) opens the door to the development of targeted therapies.
Published in the journal Nature Communications, the development caps several years of research by doctoral student Viviane Tran under the direction of Université de Montréal medical professor Dr. Jean-François Côté, the IRCM's president and scientific director, with international partners.
The formation of muscles, a complex process, requires the action of specialized cells, the myoblasts. In order for skeletal muscle to develop and regenerate, myoblasts must align with each other, move towards each other, and touch each other until their membranes are joined. This is called the myoblast fusion stage and is the basis for the formation of muscle fibers.
During embryogenesis, myoblast fusion is crucial, with mutations in certain genes resulting in the extremely rare clinical myopathy called Carey-Fineman-Ziter syndrome.
In adults, an army of satellite cells is responsible for muscle growth and regeneration. In response to activation signals, satellite cells proliferate, differentiate and fuse to repair damaged myofibers. The proteins and signaling pathways that control this fusion are still being identified.
'We didn't think it possible'
"Until recently, myoblast fusion was the subject of only basic research," said Dr. Côté.
"We weren't interested in it in the context of disease; we didn't think it was possible to use this process to cure certain diseases. Yet, understanding in detail all the factors involved in this fusion could contribute to the development of targeted therapies."
In what was a key experiment, the researchers created a mouse model in which a protein involved in fusion is expressed in its active form in the mammal. During muscle development and regeneration, an increase in myoblast fusion was observed.
"We also observed that this mouse model, when crossed with a mouse modeling limb-girdle muscular dystrophy 2B, can improve disease phenotypes," said Tran.
Direct evidence of usefulness
The new data therefore provide direct evidence that the myoblast fusion process could be exploited for regenerative purposes and to improve the outcome of muscle diseases.
In the long term, this research shows, increasing cell fusion could "repair" muscles in other types of muscular dystrophy, such as Duchenne (occurring in 1 in 4,000 boys) or other severe conditions, such as cachexia (secondary muscle breakdown due to various diseases and some forms of cancer).
The potential to manipulate the myoblast fusion step will undoubtedly be the subject of future studies, said the researchers, who worked with colleagues at UdeM's Institute for Research in Immunology and Cancer and the IRCM, in Montreal and internationally.
More information: Viviane Tran et al, Biasing the conformation of ELMO2 reveals that myoblast fusion can be exploited to improve muscle regeneration, Nature Communications (2022). DOI: 10.1038/s41467-022-34806-4
Provided by University of Montreal