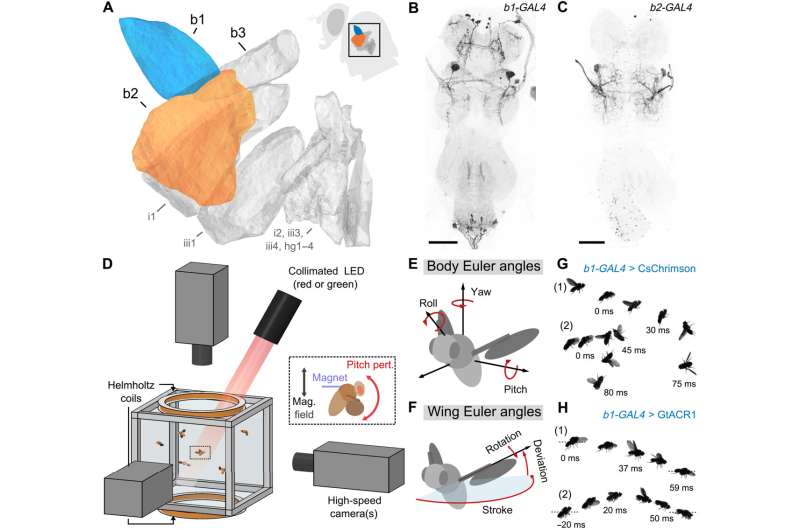
Combining genetic tools and free flight apparatus to quantify the effects of b1 and b2 manipulations. (A) Direct steering muscles of the Drosophila wing motor, with the first and second basalars (b1 and b2) highlighted in blue and orange, respectively. Data are from (30). The inset shows a fly silhouette, with a black box indicating the approximate position of the steering muscles. (B and C) Maximum intensity projections of the fly ventral nerve cord (VNC) expressing CsChrimson-mVenus (black) driven by b1-GAL4 (B) and b2-GAL4 (C). The light gray color shows DNCad (neuropil). Scale bars, 50 μm. (D) Schematic of the experimental apparatus used to deliver optogenetic and/or mechanical perturbations to freely flying Drosophila while filming their maneuvers at 8000 frames/s. The inset illustrates magnetic field from Helmholtz coils interacting with a magnetic pin glued to a fly to produce a perturbing pitch torque—data from these magnetic perturbation events are used to directly investigate the flight stabilization reflex. The optical trigger (not shown) allows automated capture of hundreds of movies per trial. (E and F) Definitions of the body (E) and wing (F) Euler angles used to describe flight kinematics. (G and H) Photomontages of example flies undergoing optogenetic excitation (G) (movies S1 and S2) and silencing (H) (movies S3 and S4) of the b1 motoneuron using CsChrimson and GtACR1, respectively. Each panel shows photomontages of two flies—labeled “(1)” and “(2)”—with time stamps indicating the timing relative to the onset of the 50-ms light-emitting diode (LED) stimulus (t = 0 ms). See table S2 for full fly genotypes. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abo7461
The flight of insects may look effortless, but as with any animal, their movements would be wildly uneven without an intricate system of neural signaling and muscle response to stabilize and steer them.
A Cornell University-led collaboration has used a combination of targeted neural manipulation and magnetic perturbance to pinpoint the two components of a fruit fly's flight stabilization system. Specifically, the researchers identified two elements of the steering muscle system responsible for the actuation of two separate control signals that enable the insect to stabilize its pitch: angular displacement and angular velocity. The finding provides evidence for an organizational principle in which each muscle has a specific function in flight control.
The group's paper, "Neuromuscular Embodiment of Feedback Control Elements in Drosophila Flight," published Dec. 14 in Science Advances.
In order to parse this complex neuromuscular system, the team—led by senior author Itai Cohen, professor of physics in the College of Arts and Sciences—studied flies that had been genetically engineered to make use of a technique called optogenetics, which installs light-sensitive channels on specific neurons. By shining a light on the fly, the researchers could turn on or off specific motor neurons, affecting the function of whatever muscle that neuron activates.
In addition, each fly had small ferromagnetic pins glued to the dorsal side of its thorax, which enabled the researchers to disrupt its flight by applying a magnetic field. The disturbance caused the fly to pitch forward or backward—essentially "tripping" the fly mid-air—and, using three high-speed cameras filming at 8,000 frames per second, the researchers captured the fly's efforts to generate a corrective torque and recover from this perturbation.
The fly's stabilization reflex begins with the haltere, a sensory apparatus that is actually the remnants of the fly's third and fourth wings, and serves as a kind of balancing organ. The haltere registers the rate of rotation of the fly's body, then sends rapid feedback through a neural circuit to the fly's 12 wing muscles that steer and stabilize it.
The researchers modeled these reflexes with what's known as a proportional-integral controller—a control loop of sorts that compensates for feedback, similar to cruise-control systems in automobiles.
"They feed the information from sensory systems to these two components of the controller, the integral part and the proportional part, which get summed together," Cohen said. "This combined signal determines, through the wing muscles, the new wing-stroke parameter that will provide a corrective aerodynamic torque, which acts on the fly body, which then acts on the sensor, providing a closed circuit."
The researchers determined the fly's b1 and b2 muscles were directly responsible for the angular displacement (the integral term) and angular velocity (the proportional term) that govern its wing forward sweep angle.
Cohen and Whitehead worked with a range of collaborators, including co-author Nilay Yapici, assistant professor of neurobiology and behavior and Nancy and Peter Meinig Family Investigator in the Life Sciences; Jesse Goldberg, associate professor of neurobiology and behavior; and Joseph Fetcho, the Dr. David and Dorothy Joslovitz Merksamer Professor of Biological Science, all in the College of Arts and Sciences.
"What's at stake is understanding how a biological system like the fly, using neurons and muscles, implements a control strategy that's ubiquitous in human-engineered systems," Whitehead said. "We're especially excited that our findings are not only a first step in that direction, but also a proof of concept for future studies that explore these neural circuits more holistically."
Co-authors include postdoctoral researcher Matt Meiselman; and researchers from Villanova University, California Institute of Technology, Johns Hopkins University and Howard Hughes Medical Institute's Janelia Research Campus.
More information: Samuel C. Whitehead et al, Neuromuscular embodiment of feedback control elements in Drosophila flight, Science Advances (2022). DOI: 10.1126/sciadv.abo7461
Journal information: Science Advances
Provided by Cornell University