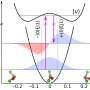
Schematic view of sub-barrier and continuum electron dynamics in strong-field ionization (a) and principles of chiral attoclock (b) and subcycle gated photoelectron interferometry (c) techniques. In (a), ionization occurs as part of the initial bound electron wave packet tunnels through the target potential barrier lowered by the strong laser field. The released electron is then subject to scattering onto the ionic potential in the continuum. In (b), randomly oriented molecules are ionized by a bicircular corotating two-color laser field E ( t ) (red continuous line). The dashed red line corresponds to − A ( t ) , the negative of the vector potential. In the SFA framework, the asymptotic photoelectron angular distribution [here displayed in the ( px , py )-polarization plane] would follow the shape of − A ( t ) and point to φ0 = 0 . Deviations from this direction can be read as attoclock offsets. In the case of a chiral target, these offsets are forward-backward asymmetric with respect to the light propagation axis z . In (c), the molecules are ionized by an orthogonal two-color laser field (red continuous line). Two electron wave packets are released per half laser cycle, creating an interference pattern in the photoelectron angular distribution. In the case of a chiral target, these interferences present asymmetric features which include information about the impact of chirality on the amplitude and phase profiles of tunneling electron wave packets. We display in (d) the Cartesian and spherical momentum coordinate systems used throughout the paper. Credit: Physical Review X (2021). DOI: 10.1103/PhysRevX.11.041056
Will an electron escaping a molecule through a quantum tunnel behave differently depending on the left- or right-handedness of the molecule?
Chemists have borrowed the phrases "left-handed" and "right-handed" from anatomy to describe molecules that are characterized by a particular type of asymmetry. To explore the concept of chirality, look at your hands, palms up. Clearly, the two are mirror images of one another. But try as we might to superimpose them, they will not overlap completely. Such objects, termed "chiral," can be found at all scales in nature, from galaxies down to molecules.
Each day, we experience chirality not only when we grab an object or put on our shoes but also when we eat or breathe: our taste and smell can distinguish two mirror images of a chiral molecule. In fact, our body is so sensitive to chirality that a molecule can be a medicine and its mirror image a poison. Chirality is thus crucial in pharmacology, where 90 percent of synthesized drugs are chiral compounds.
Chiral molecules have particular symmetry properties that make them great candidates for the investigation of fundamental phenomena in physics. Recently, the research teams led by Prof. Yann Mairesse from CNRS / Bordeaux University and Prof. Nirit Dudovich of the Weizmann Institute's Department of Physics of Complex Systems used chirality to shed new light on one of the most intriguing quantum phenomena: the tunneling process.
Tunneling is a phenomenon in which quantum particles cross seemingly impossible-to-cross physical barriers. Since this motion is forbidden in classical mechanics, it is very difficult to establish an intuitive picture of its dynamics. To create a tunnel in chiral molecules, the researchers exposed them to an intense laser field. "The electrons of the molecules are naturally bound around the nuclei by an energy barrier," explains Mairesse. "You can imagine the electrons as air trapped inside an inflatable balloon. The strong laser fields have the ability to reduce the thickness of the balloon enough for some air to tunnel through it, even though there's no hole in the balloon."
Mairesse, Dudovich and their teams set out to study an as-of-yet-unexplored aspect of tunneling: the moment in which a chiral molecule meets a chiral light field, and the way in which their brief encounter affects electron tunneling. "We were very excited to explore the connection between chirality and tunneling. We were keen to learn more about what tunneling would look like under these particular circumstances," says Dudovich.
It only takes a few hundred attoseconds for an electron to escape an atom or molecule. Such minuscule time frames characterize many of the processes studied in Mairesse's and Dudovich's labs. The two teams asked the following question: How does the chirality of a molecule affect the escape of an electron?
"We used a laser field that rotates in time to spin the barrier around the chiral molecules," says Mairesse. "To follow up with the balloon metaphor, if the laser field rotates horizontally, you expect the air to exit the balloon on the horizontal plane, following the direction of the laser field. What we found is that if the balloon is chiral, the air exits the balloon flying towards the floor or the ceiling, depending on the rotation direction of the laser. In other words, the electrons emerge from the chiral tunnel with a memory of the rotation direction of the barrier. This is very much like the effect of a corkscrew, but at the nanometer and attosecond scales."
The two teams thus discovered that the likelihood that an electron will undergo tunneling, the phase at which the electron tunnels out and the timing of the tunneling event depend on the chirality of the molecule. These exciting results lay the groundwork for additional studies that will use the unique symmetry properties of chiral molecules to investigate the fastest processes occurring in light-matter interaction.
The paper is published in the journal Physical Review X.
More information: E. Bloch et al, Revealing the Influence of Molecular Chirality on Tunnel-Ionization Dynamics, Physical Review X (2021). DOI: 10.1103/PhysRevX.11.041056
Journal information: Physical Review X
Provided by Weizmann Institute of Science
![Schematic view of sub-barrier and continuum electron dynamics in strong-field ionization (a) and principles of chiral attoclock (b) and subcycle gated photoelectron interferometry (c) techniques. In (a), ionization occurs as part of the initial bound electron wave packet tunnels through the target potential barrier lowered by the strong laser field. The released electron is then subject to scattering onto the ionic potential in the continuum. In (b), randomly oriented molecules are ionized by a bicircular corotating two-color laser field E ( t ) (red continuous line). The dashed red line corresponds to − A ( t ) , the negative of the vector potential. In the SFA framework, the asymptotic photoelectron angular distribution [here displayed in the ( px , py )-polarization plane] would follow the shape of − A ( t ) and point to φ0 = 0 . Deviations from this direction can be read as attoclock offsets. In the case of a chiral target, these offsets are forward-backward asymmetric with respect to the light propagation axis z . In (c), the molecules are ionized by an orthogonal two-color laser field (red continuous line). Two electron wave packets are released per half laser cycle, creating an interference pattern in the photoelectron angular distribution. In the case of a chiral target, these interferences present asymmetric features which include information about the impact of chirality on the amplitude and phase profiles of tunneling electron wave packets. We display in (d) the Cartesian and spherical momentum coordinate systems used throughout the paper. Credit: Physical Review X (2021). DOI: 10.1103/PhysRevX.11.041056 Electrons on run: On chirality, tunneling and light fields](https://scx1.b-cdn.net/csz/news/800a/2022/electrons-on-run-on-ch.jpg)