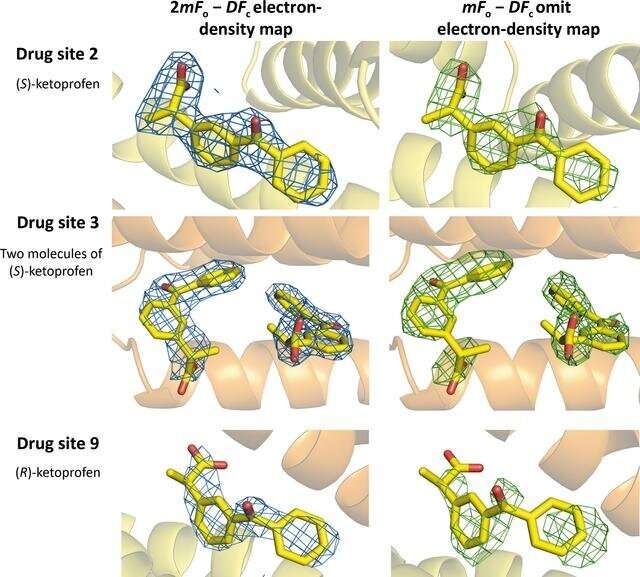
Ketoprofen binding sites in HSA (PDB entry 7jwn). The 2mFo − DFc electron-density map (r.m.s.d. of 1.0 Å) is presented in blue and the mFo − DFc omit electron-density map (map calculated after ten REFMAC refinement cycles without the drug in the model, r.m.s.d. of 2.5 Å) is presented in green. Ketoprofen molecules are shown in stick representation with O atoms in red and C atoms in yellow. Credit: IUCrJ (2022). DOI: 10.1107/S2052252522006820
A surprising discovery from the University of Virginia School of Medicine has torpedoed a key principle used in the development of new drugs to treat diseases. The finding could speed the drug-development process and help prevent potentially harmful drug interactions.
The new research from UVA's Wladek Minor, Ph.D., and collaborators calls into question an approach commonly used in drug development. Until now, scientists assumed that an important drug transporter in the blood, a protein called albumin, behaves similarly to lab models widely used in drug development as it will in human blood. But Minor's new work demonstrates that's not necessarily the case.
"Our unexpected findings should be of special interest to people studying [biological processes called] protein-ligand interactions and for scientists performing pre-clinical research in drug discovery," said Minor, Harrison Distinguished Professor in UVA's Department of Molecular Physiology and Biological Physics. "The cross-drug interaction is often discovered during clinical trials and sometimes only when new medicines are on the pharmacy shelves."
Alan Stewart, Ph.D., a co-author of the study at the University of St Andrews in the United Kingdom, said the findings offer important guidance for developing safer drugs. "This work directly demonstrates that drugs can be carried differently around the body across mammals and highlights a need for caution when using non-human albumins or animal models in drug development," he said.
Drug development insights
Serum albumin is made in the liver and serves many important functions in the body, including maintaining the proper distribution of bodily fluids in tissues. It's an important delivery vehicle, picking up substances such as hormones, fatty acids and drugs and ferrying them through the blood to where they are needed.
Serum albumin is found not just in humans but in mammals generally. As such, scientists often rely on non-human albumins, such as bovine albumin from cattle, and on animal models, such as lab mice, to predict how albumin will interact with drugs in human patients. But Minor's new work points out unexpected pitfalls of that practice.
Minor and his team looked at how a common nonsteroidal anti-inflammatory drug (NSAID), ketoprofen, interacted with both human albumin and other mammalian albumins. They discovered that these interactions were quite inconsistent, with different albumins binding with the drug in different locations. That suggests that assumptions drawn from the mammalian albumins might not produce reliable results for developing drugs for humans.
"A deeper understanding of drug transport by plasma proteins, its potential variability across species and patients, and implications for drug-drug interactions will definitely facilitate small-molecule drug discovery and ultimately benefit patients," said Ivan Shabalin, Ph.D., an alumnus of Minor's lab now working on a precision-medicine approach to oncology therapeutics at IDEAYA Biosciences in San Francisco.
Based on their findings, Minor and his collaborators are calling for additional studies scrutinizing how other drugs bind with albumin. Moving forward, they say, it will be essential for scientists to assess the suitability of particular albumins for their research. This understanding will help them better predict how human albumin will bind with the drugs and reduce the risk of unexpected and unwanted drug interactions.
"This research shows that when we are getting unexpected results, we need to dig deeply to understand them and their consequences. Advanced biomedical research often requires transdisciplinary collaboration of scientists with disparate backgrounds and perspectives to address complex research questions," Minor said. "We hope that this work will help researchers understand the limitations of pharmacological studies aimed at treating humans but that are based on other organisms."
The researchers have published their findings in IUCrJ. The paper was accompanied by an editorial highlighting the work's importance and was featured on the journal's cover. The research team consisted of Mateusz P. Czub (a UVA Ph.D. recipient now at Paul Sherer Institute in Switzerland), Stewart, Shabalin and Minor.
More information: Mateusz P. Czub et al, Organism-specific differences in the binding of ketoprofen to serum albumin, IUCrJ (2022). DOI: 10.1107/S2052252522006820
Provided by University of Virginia