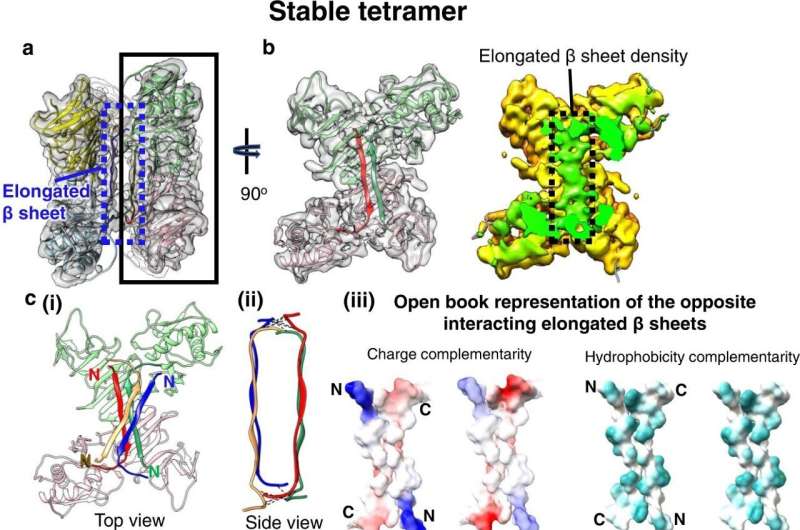
Structure of the stable sNS1 tetramer. a Two NS1 dimeric structures fitted into the cryoEM map (reduced sharpening was applied to make the density of the elongated β-sheet clearer). One dimer is colored in pink and light green and the other in yellow and light blue. b Left: the overall structure of the sNS1 dimer is similar to that of iNS1 except for the β-roll region (residues 1–30) in iNS1, which is an elongated β-sheet (residues 18–27) in sNS1. View from the elongated β-sheet of the black boxed region in a. The respective elongated β-strand of each protomer is highlighted in a darker shade of the same color (red and dark green). Right: surface density map with the regions corresponding to the elongated β-sheet colored in green, and the rest of the map in yellow. c Interaction of the elongated β-strands between the two dimers (red/green and yellow/blue). (i) Top view showing the elongated β-sheets of the red/green dimer interacting with that of the yellow/blue dimer (only its elongated β-sheet is shown). (ii) Side view showing possible hydrogen bonds or electrostatic interactions between the two elongated β-sheets from opposite dimers (dotted black lines). These interactions are identified by the distance between Cα backbone (<8 Å) and also their charge characteristics. (iii) The interacting residues are complementary in both charges and hydrophobicity. Open book representation of (ii): (Left) charge, and (Right) hydrophobicity distributions on the surface of the two elongated β-sheets. For charge, positive charges are colored in blue while negative charges are red. For hydrophobicity, cyan color indicates hydrophobic residues. d Comparison of iNS1 β-roll with sNS1 stable tetramer elongated β sheet and how it might change from the β-roll structure (dotted arrow on the iNS1 structure) into the elongated β sheet structure. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-34415-1
Dengue, a mosquito-borne tropical disease, remains a threat not just in Singapore but in Southeast Asia and beyond. Just this year, Singapore had more than 30,000 cases. Using advanced techniques in cryogenic electron microscopy, scientists from Duke-NUS Medical School and colleagues from the U.K. have obtained the first high-resolution views of an important dengue virus protein involved in the development of severe disease.
Published in Nature Communications, the team's insights may potentially inform and refine treatment strategies as well as drug and vaccine development against this endemic disease.
The team revealed that the non-structural protein 1 (NS1) can be presented outside the cell in both six- and four-unit formats and promote vascular leakage, a key contributor to dengue hemorrhagic fever. The authors also found that the four-unit format can resist antibodies binding, suggesting that this format is harder to tackle.
"These findings show how some NS1 proteins that are outside cells can escape the immune response," said Professor Lok Shee-Mei from Duke-NUS Emerging Infectious Diseases (EID) Program and senior author of the study. "When this happens in someone who has the dengue virus in their body, the individual has an increased risk of developing dengue hemorrhagic fever."
However, the study will provide the structural information to help design drugs that will be more susceptible to antibody binding.
Currently, there is one globally approved vaccine, which contains a cocktail that presents four different serotypes chimeric dengue particles, but it is only partly effective and works only on those who have been infected by the dengue virus before.
"Vaccines that stimulate a response against some, but not all, serotypes of the virus can backfire by causing the immune system to facilitate enhanced dengue entry into and the infection of circulating immune cells," said Dr. Bo Shu, first author of the study and Research Fellow with Duke-NUS' EID Program. "One possible way to make an effective vaccine is to NS1, which is produced by the dengue virus."
Prior to this study, the details of NS1's different structures had never been fully studied, adding to the challenge of creating that effective vaccine. The team took advantage of advances in cryogenic electron microscopy to construct the first high-resolution structures of the NS1 that is secreted outside virus-infected cells, called secreted NS1 (sNS1).
While intracellular NS1 is involved in dengue virus replication, which allows the virus to multiply inside cells, extracellular sNS1 makes blood vessels leak and triggers nose, skin, gum and intracranial bleeding—all symptoms of dengue hemorrhagic fever, the more severe form of dengue fever.
The high-resolution images revealed how four (tetrameric) or six (hexameric) units of NS1 interact to form either stably or loosely structured proteins. Interestingly, the scientists found that antibodies against sNS1 only attach to the loosely structured versions of the protein. This breaks them apart and ultimately reduces blood vessel leakiness. Stable tetrameric sNS1, on the other hand, was more resistant to antibody binding and would remain intact.
"Our results pave the way to investigating how these different forms of sNS1 can stimulate different pathways, leading to the complex vascular leakage that occurs in severe dengue disease," said Prof Lok, who is also NUS Provost's Chair Professor.
"Dengue is not only a global concern but remains a particular threat here in Singapore, where we have once again faced a major outbreak this year," said Professor Patrick Casey, Senior Vice-Dean for Research at Duke-NUS. "This innovative fundamental research by Prof Lok and her team yields valuable insights that can inform and refine treatment strategies and vaccine development against this endemic disease."
More information: Bo Shu et al, CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever, Nature Communications (2022). DOI: 10.1038/s41467-022-34415-1
Journal information: Nature Communications
Provided by Duke-NUS Medical School