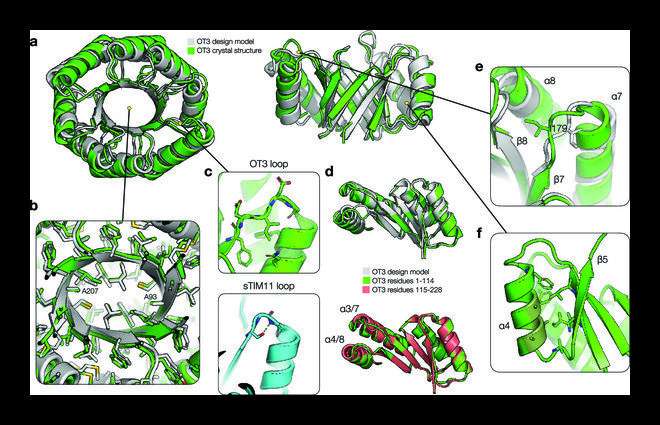
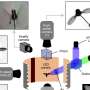
Crystal structure of OT3. (a) Comparison of OT3 design model (gray) and X-ray crystal structure (green). The top view is shown on the left, and the side view with helices 1-3 cut away is shown on the right. (b) Cutaway view of side chain packing in the hydrophobic core, with side chain heavy atoms shown as sticks. (c) Top: βα loop 3 is shown with side chains and backbone atoms and some surrounding side chains. Bottom: structural loop from sTIM11 is shown with core-facing Ser hydrogen bonded to a loop backbone atom. (d) Top: alignment of first half of OT3 structure to first half of design model. Bottom: alignment of first half of OT3 structure to second half of OT3 structure. (e) βα loop 7 shown with Ile179 packing in a different conformation compared to the design model. (f) An example of helical Ala residues forming less ideal hydrophobic cluster interactions and a lack of strong structural “knobs-into-holes” anchoring. Ala residues on α4 (dark green) and nearby side chains are shown. Credit: BioDesign Research (2022). DOI: 10.34133/2022/9842315
Proteins and enzymes perform several key functions inside the human body. To design a functional protein, it is important to be able to control the structure of protein folds and understand the relationship between the sequence, structure, and stability of proteins. Recent developments in computational biology have enabled the de novo design of proteins with varying folds and structures.
One such structure is the triosephosphate isomerase (TIM) barrel protein fold, which occurs in nearly 10% of all enzymes, and is involved in protein-mediated metabolism. It has a simple structure with repeating beta/alpha subunits that are connected by variable loops and is thus used widely as a scaffold to design other proteins. However, it has not been exploited completely to design functional proteins, due to challenges in altering its overall architecture.
Recently, a team of researchers led by Dr. Po-Ssu Huang from Stanford University conducted a study to investigate whether the structure of the central beta barrel could be altered de novo, while eliminating structural loops and improving its stability. Their goal was to design a TIM barrel protein with high stability and functional properties, and their findings were published in BioDesign Research.
"Although a TIM barrel protein has been designed de novo previously, it was difficult to finely alter the curvature of its central beta barrel, thus limiting its utility for functional design," says Dr. Huang while discussing previous attempts at creating a functional protein using the TIM barrel fold.
First, the team used the RosettaRemodel (24) framework to generate and identify ideal protein backbones using an autoregressive approach. Next, they used an iterative sequence design protocol to generate multiple sequences with a high proportion of successfully folding designs.
Together with protein synthesis and structure determination, a TIM barrel protein was developed de novo with two-fold (ovoid) symmetry and a completely new syntax, i.e., topological information, and a new sequence. The crystalline structure of this protein closely resembled the design model created by the team, confirming their design hypothesis.
Regarding the structural properties of the TIM barrel protein, Dr. Huang says, "The designed protein exhibited an elongated β barrel architecture with loops that were not structurally involved and a more developed hydrophobic core."
The team further found that the designed sequences were highly stable and were able to fold to the designed barrel curvature. In addition, the shape of the ovoid TIM barrel was found to be suitable for the incorporation of different residue identities and combinations.
Further, the team employed mutagenesis—a process wherein key amino acid residues constituting a protein get replaced with amino acids that have similar or contrasting properties. Quite astonishingly, despite the modification, the resulting TIM barrel protein displayed high structural and thermal stability, although it reduced the overall yield of the protein to a certain extent.
What are the long-term implications of these findings? "Our designs show robustness to drastic mutations, retaining high melting temperatures even when multiple charged residues are buried in the hydrophobic core or when the hydrophobic core is ablated to alanine. As a scaffold with a greater capacity for hosting diverse hydrogen bonding networks and installation of binding pockets or active sites, the ovoid TIM barrel represents a major step towards the de novo design of functional TIM barrels," says Dr. Huang.
In summary, the novel design of the TIM barrel fold has multiple implications in the field of molecular recognition and enzyme catalysis. Owing to the frequent occurrence of TIM barrel structures in key enzymes, this study also has likely therapeutic implications.
More information: Alexander E. Chu et al, De Novo Design of a Highly Stable Ovoid TIM Barrel: Unlocking Pocket Shape towards Functional Design, BioDesign Research (2022). DOI: 10.34133/2022/9842315
Provided by BioDesign Research