Biocompatible polymer protection for vaccines and drugs
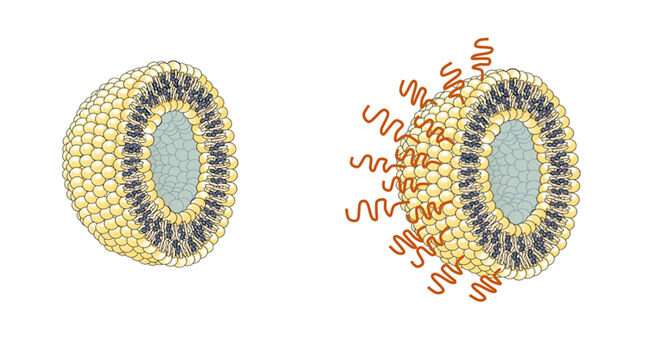
A biocompatible polymer could help deliver vaccines and drugs with reduced risk of the rare dangerous adverse reaction called anaphylaxis. Researchers at the National Institute of Advanced Industrial Science and Technology (AIST) in Japan have developed the polymer and performed preliminary tests, which they report in the journal Science and Technology of Advanced Materials.
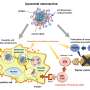
Until now, the polymer of choice for encasing and delivering vaccines has been poly(ethylene glycol) (PEG). This synthetic, flexible, water-soluble material has been used to surround some COVID-19 vaccines carried within the tiny spherical packages known as liposomes.
Unfortunately, some recipients have suffered an anaphylactic reaction to PEG, in which the immune system mounts an allergic response to the foreign material. Symptoms of anaphylaxis range from minor skin irritations, to breathing difficulty, nausea and, in the worst cases, unconsciousness and sudden death.
The alternative polymer is a form of fatty biomolecule called a lipid, and is conjugated to 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer.
This new substance spontaneously binds to the outside of liposome particles when mixed with them in water. Crucially, the polymer is not recognized by the antibodies that the body can generate in response to PEG, and tests suggest it does not stimulate any other antibodies that could cause an allergic reaction. This should allow coated liposomes containing a vaccine to be retained in the body for a longer time without being cleared by the immune system, in addition to avoiding anaphylaxis.
"We have also found that the polymer avoids other interactions with proteins in the blood that might otherwise interfere with its effects, and it also prevents liposomes from aggregating together," says molecular engineer Yuji Teramura of the AIST team.
Tests confirm the coated liposomes can remain stable in storage for 14 days, sufficient for real clinical applications.
"All the indications suggest that our technology should be suitable for delivering vaccines into patients who develop anaphylaxis in response to PEG," Teramura concludes.
The polymer must now be thoroughly tested in various real vaccine applications. The team is moving into this next crucial phase of the development process, prior to eventual clinical trials in humans.
Provided the animal and subsequent clinical trials go well, the technology should offer opportunities for delivering drugs into the body, in addition to vaccines. Delivery systems such as liposomes are sometimes needed to protect drugs from biochemical processes that might degrade them. This can ensure that they reach the target disease tissues while remaining in their active form.
More information: Haruna Suzuki et al, Impact of spontaneous liposome modification with phospholipid polymer-lipid conjugates on protein interactions, Science and Technology of Advanced Materials (2022). DOI: 10.1080/14686996.2022.2146466
Journal information: Science and Technology of Advanced Materials
Provided by Science and Technology of Advanced Materials