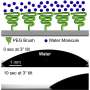
Time sequence of a shearing experiment at T = 230 K. (a-c) A hydrophobic slider (θ = 120◦) barely one nanometer thick slips past the substrate. (d-f) A hydrophilic slider (θ = 50◦) sticks to the substrate and exhibits significant frictional melting. Credit: Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2209545119
In contact with a solid the surface of ice melts, forming a lubricant layer which is self-perpetuating, as greater weight and slippage are applied to it. This cooperative phenomenon makes the ice more slippery and more likely to cause skating or car accidents, according to international research led by the Complutense University of Madrid (UCM).
In this study, published in Proceedings of the National Academy of Science, the researchers conducted a computer simulation of how a solid slides over the surface of the ice at the atomic scale.
"Our analysis of how the ice molecules are collectively organized to give them their peculiar lubricant power offers us a privileged insight into the process that could not be achieved through conventional experiments, given the huge difficulty in conducting an experimental observation of a lubricating layer of a thickness of a billionth of a meter," says Luis González MacDowell, a researcher at the UCM Physical Chemistry Department.
The slippery properties of ice have in some cases been exploited for leisure purposes (such as in ice-skating), and in others as a means of transport.
"It is important to understand the origin of this widely known property of ice, both in order to improve the performance of Olympic athletes, and to ensure vehicle safety during the winter," the expert indicates.
Movie 1. Sliding with slip at a hydrophobic wall. Movie displays the first 0.5 ns of a simulation at p=1 atm and T=262 K where a hydrophobic substrate with θ = 120° slides at a velocity of U = 5 m/s. Notice how the wall slips past the premelting film, as illustrated by the blue tagged molecules. Movie 2. Sliding with stick at a hydrophobic wall. Movie displays the first 0.5 ns of a simulation at p=1 atm and T=262 K where a hydrophilic substrate with θ = 50° slides at velocity of U = 5 m/s. Notice how the the wall-adsorbed layer sticks to the wall and moves at equal speed, as indicated by the blue tagged moelcules. Movie 3. Frictional melting of ice. Movie displays the full 10 ns of a simulation at p=1 atm and T=230 K where a hydrophilic substrate with θ = 50° slides at velocity of U = 5 m/s. Notice how the first ice bilayer melts already at 0.5 ns and an additional bilayer has melted after 5 ns. Credit: Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2209545119
Aside from the UCM, the study also involves the Autonomous University of Madrid (UAM) and Marie Curie-Skłodowska University (MCSU) of Lublin, Poland.
Compatible hypotheses which pave the way for energy savings
Scientists have spent two centuries wondering why ice is slippery, and what causes the liquid layer which forms on top of it. Over the decades, figures including Michael Faraday, James Thomson, Osborne Reynolds and Philip Browden have come up with divergent hypotheses.
However, this study has served to demonstrate that they are in truth compatible, and operate simultaneously. "What we in fact find is that the key principles of the slippery nature of ice are the surface melting phenomenon proposed by Faraday; the gradual melting caused by pressure, reminiscent of Thomson's hypothesis, and the melting caused by friction, as proposed by Bowden," the UCM chemist points out.
This combination of factors gives the surface of the ice an exceptional self-repairing lubrication layer. "The problem with lubrication is that as the pressure increases, the lubricant is expelled from between the opposing faces, which leaves them in direct contact. In the case of ice, Le Chatelier's principle operates, and as the lubricating layer is driven away by the pressure, the ice itself melts and repairs the loss," indicates Lukasz Baran, the MCSU researcher who worked on the simulation technique during a six-month placement at the UCM.
Aside from preventing sporting and traffic accidents, the results of this study could be applied in designing better lubricants in other systems.
"It is important to remember that more than half the energy generated worldwide is lost through friction. Improved lubrication processes would mean a huge saving in fuel, money and environmental impact," concludes Pablo Llombart, researcher at the UAM's Nicolás Cabrera Institute.
More information: Łukasz Baran et al, Ice friction at the nanoscale, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2209545119
Journal information: Proceedings of the National Academy of Sciences
Provided by Universidad Complutense de Madrid