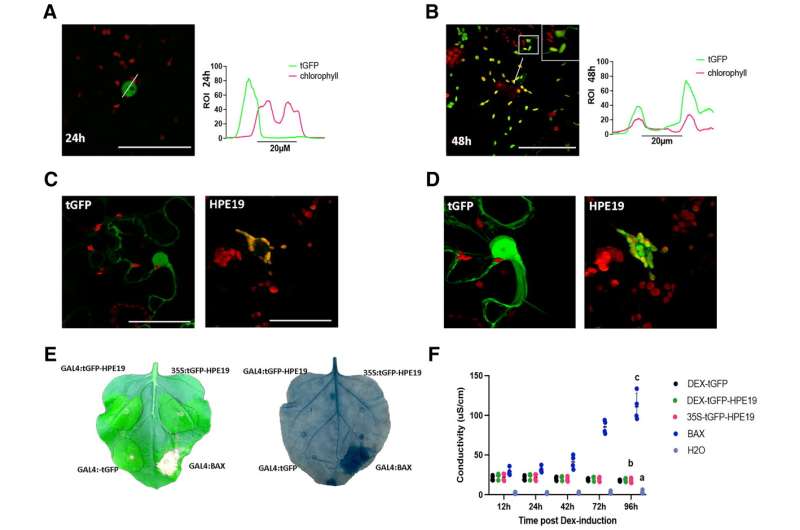
HPE19 exhibits dynamic nuclear chloroplast localization and alters immune responses in Nicotiana benthamiana. HPE19 lacking its signal peptide was cloned with an N-terminal fusion to TurboGFP (tGFP) under control of the GAL4 dexamethasone (Dex)-inducible promoter, transiently expressed in Nicotiana benthamiana and were visualized by confocal microscopy. Dex was applied 12 postinfiltration (hpi). A, HPE19 exhibits nuclear expression at 24 hpi. The left panel shows green fluorescent protein (GFP), the central panel includes chloroplast autofluorescence, and the right panel shows the intensity profile for tGFP and chlorophyll in the merged image. B, HPE19 primarily exhibits chloroplast localization at 48 hpi. Insert in the central panel shows chloroplast stromule-like projections. Right panel, tGFP and chlorophyll intensity profiles overlap in the merged image. C, Representative image of nuclei expressing tGFP (left) and chloroplasts surrounding nuclei carrying tGFP-HPE19 (right). D, Three-dimensional (3D) reconstructions of the tGFP control (left) and tGFP-HPE19 (right) 48 hpi illustrate chloroplast clustering around nuclei. 3D reconstructions were generated from 20 images. E, Overexpression of HPE19 fails to induce cell death. Left, macroscopic cell death observed 5 days postinduction with 2 μM Dex; right, cell-death visualized after trypan blue staining observed 5 days postinduction with 2 μM Dex F, Electrolyte leakage 12 to 96 hpi with 2 μM Dex. Individual dots represent values for four biological replicates per construct. Statistical differences were detected by a repeated measures analysis of variance, and different groups were detected using a Tukey multiple comparison test. Different letters indicate significantly different groups of means at 96 hpi. Credit: Molecular Plant-Microbe Interactions® (2022). DOI: 10.1094/MPMI-05-22-0114-R
Sometimes the most niche plant pathogens pack the greatest punch. Such is the case for the Florida citrus industry, which has seen a 70% decline in its orange production since the introduction of Huanglongbing (citrus greening) in 2005.
This disease is caused by the bacteria Candidatus Liberibacter asiaticus, which spreads via a flying insect—unlike most bacterial plant pathogens. When the insect feeds on the sugary sap of a plant, it deposits the bacteria into the veins of the plant, directly into the phloem, which allows the bacteria to follow this transport highway throughout the plant.
A close relative of the citrus greening pathogen, Candidatus Liberibacter solanacearum (CLso), is a newly emerging pathogen of tomato and potato. As this bacterium cannot survive outside of its hosts, very little is known about it, including how it causes disease. A recent study led by Paola Reyes Caldas, of the University of California, Davis, has discovered and characterized secreted proteins from the pathogen CLso. These proteins, called effectors, offer clues into the manipulation tactics this bacterium uses to subdue its plant host.
Newly published in Molecular Plant-Microbe Interactions, the study found that these effectors can be present in both the plant and insect host. Once inside the plant, these effectors can target various parts of the cell such as the iconic chloroplast, which are critical for the plant to perform photosynthesis.
Additionally, these effectors are mobile in that they can travel from one plant cell to another. Corresponding author Gitta Coaker comments, "These effectors can also move from cell to cell, which could explain how Liberibacter can manipulate the plant while remaining restricted to the phloem. Unlike effectors from culturable leaf colonizing bacteria, the majority of Liberibacter effectors do not suppress plant immune responses, indicating that they possess unique activities."
Whether these unique activities alter the phloem environment or insect attractiveness to facilitate pathogen spread remains to be seen, but this research offers an exciting starting point to unraveling this complex disease. Once targets of these effectors are identified, genetically engineering these important crops to prevent manipulation could be a fruitful solution to managing these diseases.
More information: Paola A. Reyes Caldas et al, Effectors from a Bacterial Vector-Borne Pathogen Exhibit Diverse Subcellular Localization, Expression Profiles, and Manipulation of Plant Defense, Molecular Plant-Microbe Interactions (2022). DOI: 10.1094/MPMI-05-22-0114-R
Journal information: Molecular Plant-Microbe Interactions
Provided by American Phytopathological Society