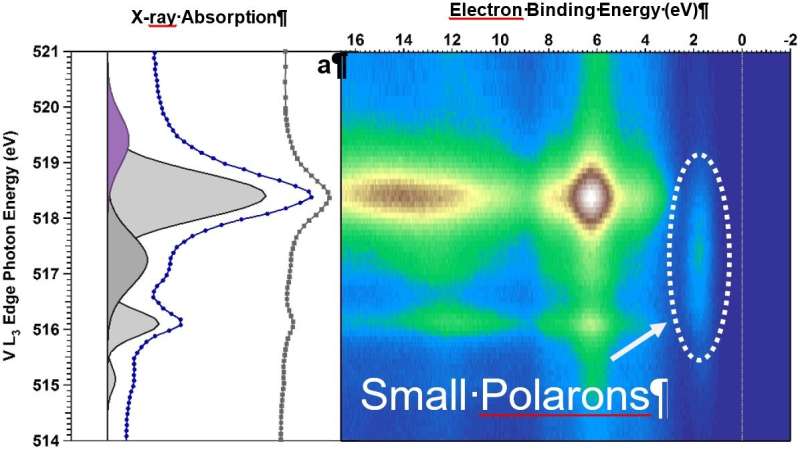
2D map of the valence band states (x-axis in eV) in Mo-doped BiVO4 as a function of photon energy (y-axis). The presence of small polarons can be deducted from the spot at approx. 2 eV. Credit: Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c07501
Photoelectrodes based on BiVO4 are considered top candidates for solar hydrogen production. But what exactly happens when they come into contact with water molecules? A study in the Journal of the American Chemical Society has now partially answered this crucial question: Excess electrons from dopants or defects aid the dissociation of water which in turn stabilizes so-called polarons at the surface. This is shown by data from experiments conducted at the Advanced Light Source at Lawrence Berkeley National Laboratory. These insights might foster a knowledge-based design of better photoanodes for green hydrogen production.
Every green leaf is able to convert solar energy into chemical energy, storing it in chemical compounds. However, an important sub-process of photosynthesis can already be technically imitated—solar hydrogen production: Sunlight generates a current in a so-called photoelectrode that can be used to split water molecules. This produces hydrogen, a versatile fuel that stores solar energy in chemical form and can release it when needed.
Photoelectrodes with many talents
At the HZB Institute for Solar Fuels, many teams are working on this vision. The focus of their research is on producing efficient photoelectrodes. These are semiconductors that remain stable in aqueous solutions and are highly active: Not only can they convert sunlight into electrical current, but they may also act as catalysts to accelerate the splitting of water. Among the best candidates for inexpensive and efficient photoelectrodes is bismuth vanadate (BiVO4).
"Basically, we know that just by immersing bismuth vanadate in the aqueous solution the chemical composition of the surface changes," says Dr. David Starr of the HZB Institute for Solar Fuels. And his colleague Dr. Marco Favaro adds, "Although there are a great many studies on BiVO4, it has not been clear until now exactly what implications this has on the surface electronic properties once they come into contact with the water molecules." In this work, they have now investigated this question.
They studied single crystals of BiVO4 doped with molybdenum under water vapor with resonant ambient pressure photoemission spectroscopy at the Advanced Light Source at Lawrence Berkeley National Laboratory. A team led by Giulia Galli at the University of Chicago then performed density functional theory calculations to help interpret the data and to untangle the contributions of individual elements and electron orbitals to the electronic states.
Polarons on the surface detected
"In situ resonant photoemission has allowed us to understand how the electronic properties of our BiVO4 crystals changed upon water adsorption," Favaro says. The combination of measurements and calculations showed that due to excess charge, generated by either doping or defects on certain surfaces of the crystal, so-called polarons may form: negatively charged localized states, where water molecules can easily attach and then dissociate.
The hydroxyl groups formed via water dissociation help to stabilize further polaron formation. "The excess electrons are localized as polarons at VO4 units on the surface," Starr summarizes the results.
"What we can't yet assess for sure is what role the polarons play in charge transfer. Whether they promote it and thus increase efficiency or, on the contrary, are an obstacle, we still need to figure that out," Starr admits. The results provide valuable insights into processes that modify the surface chemical composition and electronic structure and might foster the knowledge-based design of better photoanodes for green hydrogen production.
The research is published in the Journal of the American Chemical Society.
More information: Wennie Wang et al, Influence of Excess Charge on Water Adsorption on the BiVO4(010) Surface, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c07501
Journal information: Journal of the American Chemical Society
Provided by Helmholtz Association of German Research Centres