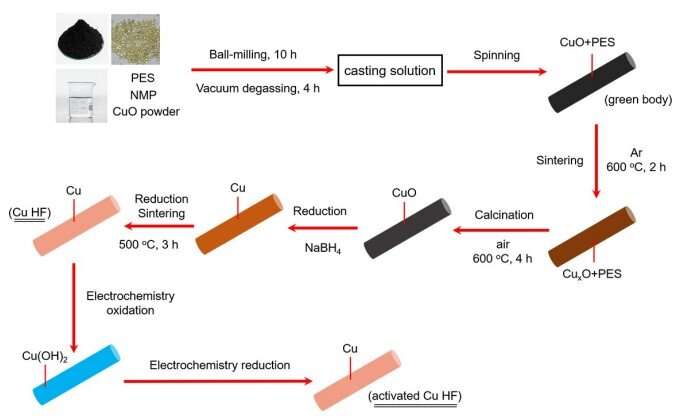
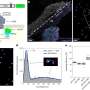
Schematic illustration showing the general procedures for the fabrications of Cu HF and activated Cu HF. Credit: Energy & Environmental Science (2022). DOI: 10.1039/D2EE02121H
Electrochemical conversion of CO2 into value-added chemical fuels driven by renewable electrical energy is a prospective strategy for addressing both CO2 emissions and energy consumption. However, the current density of CO2 to multicarbon products remains a challenge for sustained industrial-scale implementation.
Recently, a research team from the Shanghai Advanced Research Institute of the Chinese Academy of Sciences reported a hierarchical micro/nanostructured Cu(100)-rich hollow-fiber gas penetration electrode (GPE) for electrochemical reduction of CO2 to multicarbon products under ampere level current density. The electrode broke through the bottleneck of low CO2 solubility limit.
The results were published in Energy & Environmental Science on Nov. 2.
The Cu GPE is composed only of metallic copper for electrochemical CO2 reduction reaction to C2+ products. It reduced CO2 to C2+ products with a faradaic efficiency of 62.8% and a current density of 2.3 A cm-2 in 0.5 M KHCO3 solution at -1.94 V, approximating to or even outperforming state-of-the-art Cu-based catalysts.
Electrochemical results showed that optimized mass transfer and enhanced three-phase interface reaction synergistically promoted CO2 activation and reduction kinetics. Theoretical calculations further suggested that the Cu(100) facet of Cu GPE favored CO adsorption and dimerization, thus enhancing its catalytic activity.
More information: Chang Zhu et al, Ampere-level CO2 reduction to multicarbon products over a copper gas penetration electrode, Energy & Environmental Science (2022). DOI: 10.1039/D2EE02121H
Journal information: Energy & Environmental Science
Provided by Chinese Academy of Sciences