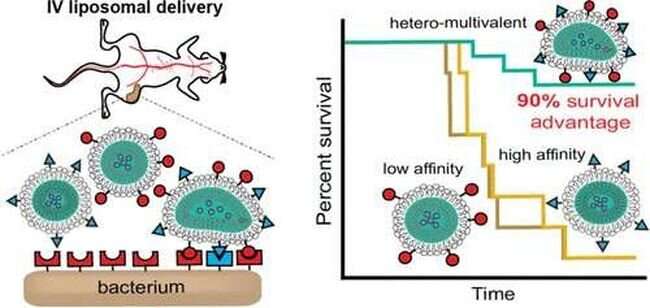
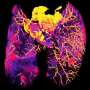
Graphical abstract. Credit: ACS Applied Materials & Interfaces (2022). DOI: 10.1021/acsami.2c12943
Hung-Jen Wu, associate professor in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, is working to defeat bacteria that have become resistant to multiple types of antibiotics. To achieve interdisciplinary results, Wu is collaborating with researchers in the Texas A&M College of Engineering and the Texas A&M Health Science Center.
"These bacteria are not just drug-resistant, they are multidrug-resistant," Wu said. "This means several different classes of antibiotics cannot kill them. This has become a really big threat to public health because doctors are unable to effectively treat these diseases with the same medications that were used in the past."
The research has been published in Advanced Therapeutics and ACS Applied Materials & Interfaces.
In the past, the medical research community has attempted to defeat antibiotic-resistant bacteria by developing new antibiotics. This approach is expensive and can take decades to accomplish. Wu is taking a different approach. By using a targeted system, he is delivering the antibiotics directly to the bacteria, thus allowing them to have a greater impact on the pathogen.
"By using a targeted delivery system, we can actually reduce the dose of the antibiotic and still effectively kill the pathogen," Wu said.
The idea to use a targeted approach emerged when researchers observed the behavior of the pathogens they were seeking to kill. When bacteria enter the body, they use a combination of strong and weak ligand connections to attach to a cell membrane and infect the cell. Ligands, which are molecular connections that exist on both the bacteria and cell membrane, allow the two cells to stick to each other by using unique molecular structures. Wu and his collaborators are exploiting this characteristic of bacteria by using this binding method to efficiently deliver antibiotics directly to the bacteria.
"We noticed how bacteria attached to the host cell's strong and weak ligands simultaneously, and we wondered how we could use that," Wu said. "We were inspired by the bacteria to develop this new drug carrier system."
While the medical community has tried to use these connections before, the effectiveness was low because researchers focused only on strong ligand pairs.
"What makes our laboratory unique is our focus on the weak ligand pairs," Wu said. "While strong ligand pairs are important, researchers had trouble finding them on the cell membrane because they are scarce. The abundance of weak ligand pairs makes them more effective to deliver medication to the cell."
To facilitate the targeted delivery, Wu has engineered molecular ligand structures on the outside of antibiotic particles that bind with the weak ligand pairs on the bacteria cell membrane. When an antibiotic particle encounters a bacteria cell, the abundance of weak ligand pairs causes the antibiotic particle to adhere to the cell in multiple locations, creating a more stable connection. This allows for more effective absorption of the antibiotic by the bacteria cell. This quick absorption of the antibiotic leads to a more effective destruction of the pathogen.
Wu's research has focused on two types of bacteria, the bacterium that causes Tuberculosis, Mycobacterium tuberculosis, and one of the leading bacterial pathogens which has displayed antibiotic resistance, Pseudomonas aeruginosa. In the future, he hopes to apply his method to additional types of bacteria to help slow the spread of other infectious diseases.
"This mechanism was designed to work with all types of bacteria," Wu said. "When we apply it to other types of bacteria, I can quickly identify the ligand pairs. We will be able to effectively treat many different types of bacteria with this new tool."
More information: Anirudh Gairola et al, Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages, Advanced Therapeutics (2022). DOI: 10.1002/adtp.202100193
Akshi Singla et al, Hetero-Multivalent Targeted Liposomal Drug Delivery to Treat Pseudomonas aeruginosa Infections, ACS Applied Materials & Interfaces (2022). DOI: 10.1021/acsami.2c12943
Journal information: ACS Applied Materials and Interfaces
Provided by Texas A&M University