'Nanoreactor' grows hydrogen-storage crystals
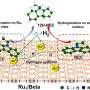
Neutron scattering techniques were used as part of a study of a novel "nanoreactor" material that grows crystalline hydrogen clathrates, or HCs, capable of storing hydrogen. The researchers, from ORNL and the University of Alicante, or UA, were inspired by nature, where methane hydrates grow in the pores and voids within natural sediments.
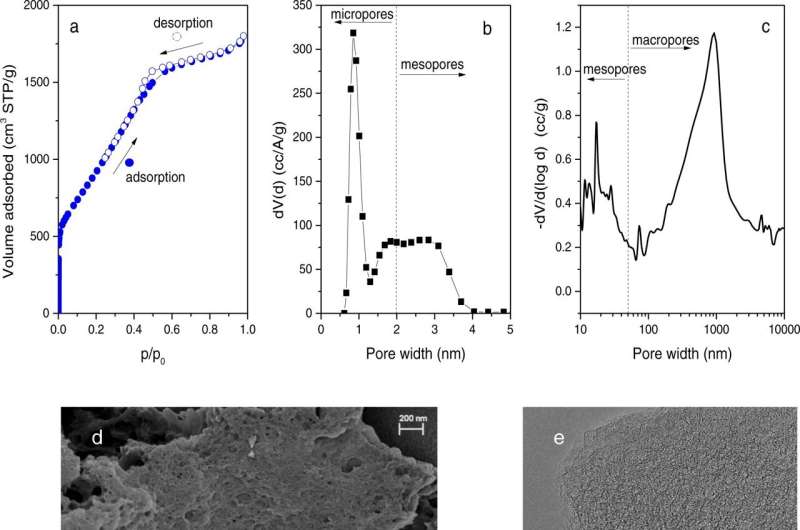
The nanoreactor material consists of a chemically optimized, porous activated carbon that can confine hydrogen at the nanoscale with excellent thermal stability as high as -27.7 degrees Fahrenheit. Pure liquid water, without additives, is all that is needed to promote HC formation. Nearly 100% of the water is converted into HCs in just minutes—at a 30% lower pressure than required in conventional HC production.
"The ability to store hydrogen at lower pressures and higher temperatures is a step toward potentially using these crystalline hydrates for hydrogen storage in stationary and mobile applications," said UA's Joaquin Silvestre-Albero.
The study appears in Nature Communications.
More information: Judit Farrando-Perez et al, Rapid and efficient hydrogen clathrate hydrate formation in confined nanospace, Nature Communications (2022). DOI: 10.1038/s41467-022-33674-2
Journal information: Nature Communications
Provided by Oak Ridge National Laboratory