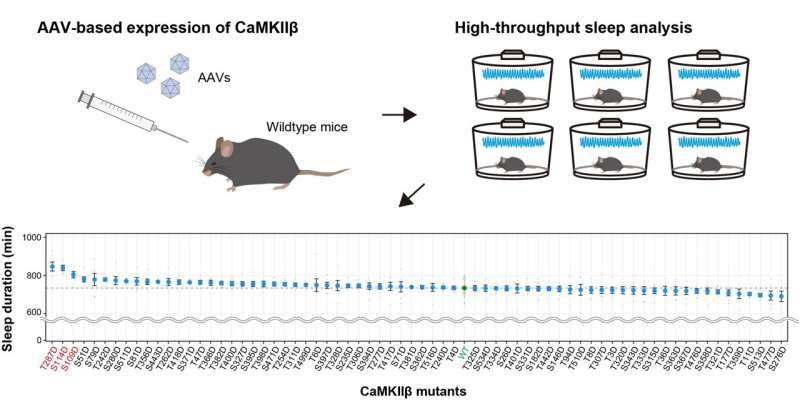
Fig.1 AAV-based screening for CaMKIIβ phosphomimetic mutants Wild-type mice were administered with AAVs (adeno-associated viruses) to express the CaMKII mutant (upper right) and their sleep phenotypes were analyzed (upper left). Comprehensive analysis of mice administrated with 69 AAVs corresponding to CaMKIIβ phosphomimetic mutants revealed that T287D (T287D), a phosphomimetic mutant of T287, remarkably extended sleep duration (below). Credit: Tone et al.
Professor Hiroki Ueda, Assistant Professor Daisuke Tone, Lecturer Koji Ode, and Qianhui Zhang (4th-year graduate student at the time) at the University of Tokyo showed that CaMKIIβ, a predominant protein kinase expressed in neurons, is involved in prolonging sleep duration via facilitating sleep onset and inhibiting awakening.
Several recent studies have shown that protein phosphorylation is important for sleep regulation. The aforementioned study group reported in 2016 that CaMKIIα and CaMKIIβ are protein kinases that promote sleep and suggested the possibility that protein phosphorylation is the likely molecular driver of somnolence and proposed phosphorylation hypothesis of sleep. CaMKIIα and CaMKIIβ are abundantly expressed in brain nerve cells and have been extensively studied to show their important role in functions such as memory. However, the functions and conditions of CaMKIIα and CaMKIIβ in sleep control were unknown.
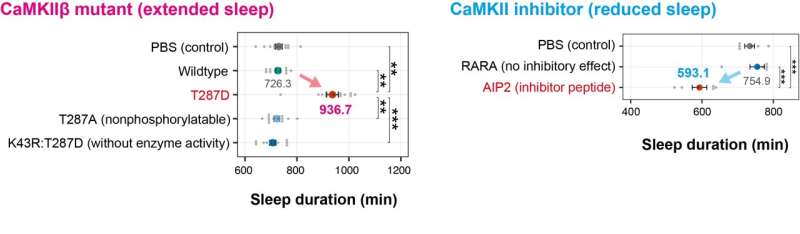
Fig.2 Bidirectional regulation of sleep by CaMKII The T287D mutant extends sleep duration, while mutations that do not act as phosphomimetics (T287A), and a mutation that causes loss of enzyme activity (K43R), indicating that the T287 phosphorylation and CaMKII enzyme activity is important for sleep regulation (left). Expression of a CaMKII inhibitor peptide resulted in a shortened sleep duration (right). Credit: Tone et al.
It is well-known that CaMKIIβ has multiple phosphorylation sites and that its function is modulated according to their phosphorylation status. Therefore, this study group generated a series of CaMKIIβ mutants. Toward this, mutated residues that mimic the phosphorylation state were introduced at each site, and the relationship between CaMKIIβ phosphorylation state and sleep regulation was comprehensively investigated using mice. The researchers found that in specific phosphorylation state, CaMKIIβ promoted the transition from wakefulness to sleep state. Furthermore, when an additional second or third similarly phosphorylated variant was introduced into CaMKIIβ in this specific phosphorylated state, transition from the sleep state to wakefulness was inhibited.
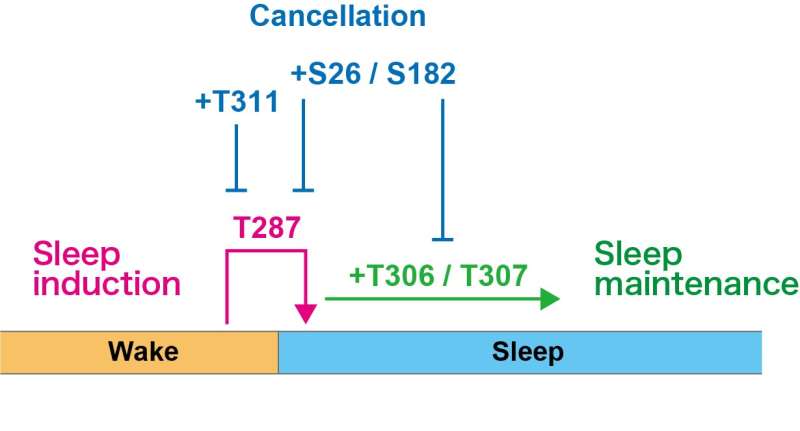
Fig.3 A schematic model of sleep regulation by CaMKIIβ phosphorylation state CaMKIIβ acts on induction (T287), maintenance (T306, T307), and their cancellation (S26, S182, and T311) of sleep, depending on its own phosphorylation state. Credit: Tone et al.
In the present study, now published in PLOS Biology, they found that CaMKIIβ functions at each step of a multi-step process that prolongs sleep duration (induction and maintenance of sleep) depending on its state of phosphorylation. Many existing hypnotics act on the steps involved in the induction of sleep. This study partly clarified the mechanism underlying sleep induction and maintenance and provides clues to consider more ideal sleep control methods aimed at maintaining sleep, which has been difficult so far.
This result was obtained as part of the JST Exploratory Research for Advanced Technology (ERATO) under the Ueda Biological Timing Project. In this project, JST developed an approach based on systems biology contributing to human understanding using sleep–wake rhythms as a model. The aim was to obtain information pertaining to biological timing that extends from molecules to individuals, in terms of human sleep–wake cycle.
More information: Daisuke Tone et al, Distinct phosphorylation states of mammalian CaMKIIβ control the induction and maintenance of sleep, PLOS Biology (2022). DOI: 10.1371/journal.pbio.3001813
Journal information: PLoS Biology
Provided by Japan Science and Technology Agency