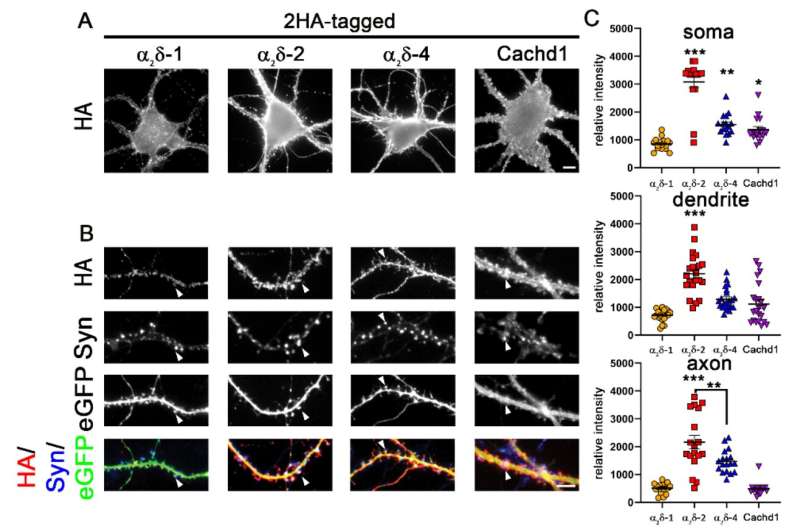
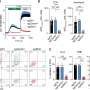
α2δ-4 and Cachd1 can be expressed on the somato-dendritic and axonal surface. (A) Live cell surface staining of wildtype primary hippocampal neurons overexpressing soluble eGFP together with HA-tagged constructs of α2δ subunits or Cachd1. (A) The somatic expression pattern of the anti-HA labeling revealed that all α2δ subunits and Cachd1 can be expressed on the neuronal surface. (B) Anti-HA and synapsin immunofluorescent labeling revealed that all α2δ subunits and Cachd1 show synaptic and dendritic membrane targeting (white arrowheads). Neurons overexpressing α2δ-1 displayed a weaker expression pattern on the somatic, dendritic, and axonal surface compared to α2δ-2, α2δ-4, and Cachd1. (C) Quantification of the average HA fluorescent intensities illustrated that the surface expression of α2δ-1 was reduced compared to those of the other subunits. α2δ-2 expression in soma, dendrites, and axons was higher compared to α2δ-1, α2δ-4, and Cachd1. Quantification data are shown as values for individual cells (dots) and means ± SEM. ANOVA with Tukey’s multiple comparison test (C) 16–22 cells per condition from three independent culture preparations. F(3, 60) = 53.6, p < 0.0001. Significances of post hoc test (*** p < 0.001, ** p < 0.01, * p < 0.05) in comparison to α2δ-1, unless otherwise indicated, are indicated with asterisks. Scale bars, 5 µm. Credit: International Journal of Molecular Sciences (2022). DOI: 10.3390/ijms23179885
Five proteins share important roles in the formation and function of synapses and can substitute for each other. This discovery was made by a team of the Karl Landsteiner University of Health Sciences Krems (KL Krems) and the CavX Ph.D. program of the Medical University of Innsbruck, and their work is now published in the International Journal of Molecular Sciences.
Most of these proteins are components of so-called calcium channels, and only recently, the team discovered redundant functions for three of the proteins in synapse formation and neuronal signal transmission. The recent finding that two additional proteins (α2δ-4 and Cachd1) can fulfill the same functions is surprising and raises questions about the evolution of the nervous system.
Ion channels serve to conduct signals in the nervous system, so it is essential that their function is tightly regulated. Proteins of the α2δ-family play an important role in this process. They serve as regulatory subunits of calcium channels and hence for a long time they are known to regulate calcium currents.
Recently, however, Univ. Prof. Dr. Gerald Obermair, head of the Division of Physiology at the KL Krems, and his team were able to show that three of the four α2δ-proteins are also chiefly involved in the formation of synapses and that they can substitute for each other in this fundamental function.
This caused a considerable stir, as α2δ-proteins are associated with diseases such as epilepsy, autism, schizophrenia, and anxiety. Now Prof. Obermair and his research group have succeeded in showing that the last of the four proteins of this family and also another protein not only influence synapse formation, but also affect signal transmission.
Two is better, many are best
In contrast to the previously studied α2δ-proteins (isoforms -1, -2, -3), the now investigated α2δ-4 occurs predominantly in the retina of the eye but is hardly found in the brain. The current results, as Prof. Obermair explains, are even more surprising: "We were able to show in cell cultures that α2δ-4 can exert very similar functions in the brain as the previously studied proteins α2δ-1 to -3. Indeed, all these proteins can even replace each other in their most critical function. This seems wasteful and is remarkable in evolutionary terms."
On top of that, the team also studied a protein known as Cachd1. While this protein is structurally similar to the α2δ-proteins, it is still unclear whether it also serves as a subunit of ion channels. Unlike α2δ-4, however, it is abundantly found in the brain and has been linked to brain functions. This and its similarity to α2δ-proteins were reasons enough to take a closer look at the functions of Cachd1.
"And indeed," elaborates Cornelia Ablinger, first author of the study and a student in the CavX Ph.D. program, "it turned out that Cachd1 can also take over the functions of α2δ-proteins. Hence, it can modulate synapse formation and also affect channel function."
Further experiments with all α2δ isoforms and Cachd1 showed that the ability to substitute for each other does not come without subtle differences. For example, analyses of synaptic calcium signals identified minute differences indicating specific modulatory roles of each protein. A finding that allows Prof. Obermair to speculate on the apparent redundancy of the proteins: "It may well be that in the course of evolution they diversified one after the other to adapt the critical control of nerve signal transmission to the requirements of increasingly complex organisms."
Experimental challenge
The surprising results were only made possible by ten years of preliminary work by Prof. Obermair's team. Actually, it was the ability of α2δ proteins to substitute for each other that posed an experimental challenge. Particularly, because first a cellular nerve cell model had to be developed in which all three genes for α2δ-1 to -3 were switched off. This endeavor turned out to be a big challenge, with a success rate of less than 5%.
However, once the team—which received a great deal of international attention—had overcome this hurdle, the questions could be experimentally tackled. The discovery that α2δ-4 and Cachd1 can also modulate synapse formation and differentiation was made possible by the preliminary work. Precise measurements of calcium signals of individual synapses provided evidence that α2δ-4 and Cachd1 can also modulate channel function.
More information: Cornelia Ablinger et al, α2δ-4 and Cachd1 Proteins Are Regulators of Presynaptic Functions, International Journal of Molecular Sciences (2022). DOI: 10.3390/ijms23179885
Provided by Karl Landsteiner University of Health Sciences