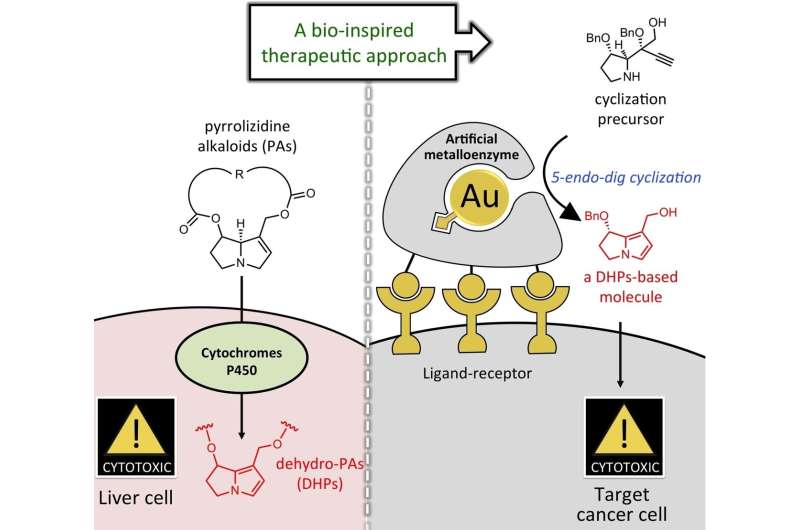
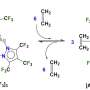
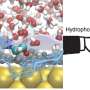
Graphical abstract. Credit: Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202205541
Researchers in Japan have succeeded in inhibiting cancer cell growth using pyrrolizidine alkaloid, a component of plant origin previously thought to be too toxic to administer.
Pyrrolizidine alkaloids are found in about 6,000 plant species, including the daisy and bean families. In plants, they protect plants from predators; however, in humans, they have antibacterial and antitumor properties, making them useful for herbal medicine. Early studies reported pyrrolizidine alkaloids could kill cancer cells, but the research was abandoned because they also caused liver damage.
"The problem of being toxic to the liver is inseparable from the mechanism of activity of pyrrolizidine alkaloids," lead author Professor Satoshi Yokoshima explains. "Pyrrolizidine alkaloids only show toxicity when metabolized in the body and converted to their 'active form' containing the pyrrole structure. The active form damages the cancer cell's DNA, preventing it from reproducing, but it also makes them harmful to nucleic acids and proteins in the liver."
Professor Yokoshima of the Graduate School of Pharmaceutical Science, Nagoya University, together with Professor Katsunori Tanaka of the Tokyo Institute of Technology and RIKEN, investigated the possibility of inhibiting cancer cell proliferation without toxicity. The researchers administered a molecular precursor of pyrrolizidine alkaloid with a different structure. Then, they converted it to the active body containing the pyrrole structure near cancer cells to minimize damage. Their findings were published in Angewandte Chemie International Edition.
"I am interested in the structure of natural products," Professor Yokoshima explains. "It is fun to synthesize them. We may see something new in organic chemistry or develop a new method. In this experiment, we designed a new compound with a different structure as a precursor to the active form. Since it did not harm the body, we introduced it and then converted it to the active form using a gold catalyst in the presence of cancer."
To introduce gold catalysts into the body, the team turned to Professor Tanaka from RIKEN who had supported a gold catalyst on albumin, a protein found in blood. They also introduced multiple sugar chains to the surface of albumin, taking advantage of the fact that sugar chains recognize the surface of cancer cells. The albumin bound to the cancer cells and reaction occurred close to cancer cells, limiting the damage to other cells.
The team confirmed the conversion to the active form containing the pyrrole structure. This was evidence of "on-site synthesis," which means the active form was created near cancer cells to limit damage to the body. They also confirmed remarkable growth inhibition of the targeted cancer cells.
"Pyrrolizidine alkaloids are toxic to the liver, but this method can avoid toxicity," says Professor Yokoshima. "If we can apply this in vivo, it could be a new method of cancer treatment. We hope that other potential anticancer treatments that were discontinued because of toxicity problems can be tested again as potential treatments using the on-site synthesis method. I hope this method will offer insights others can use to make their own drugs."
More information: Michitaka Kurimoto et al, Anticancer Approach Inspired by the Hepatotoxic Mechanism of Pyrrolizidine Alkaloids with Glycosylated Artificial Metalloenzymes, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202205541
Journal information: Angewandte Chemie International Edition
Provided by Nagoya University