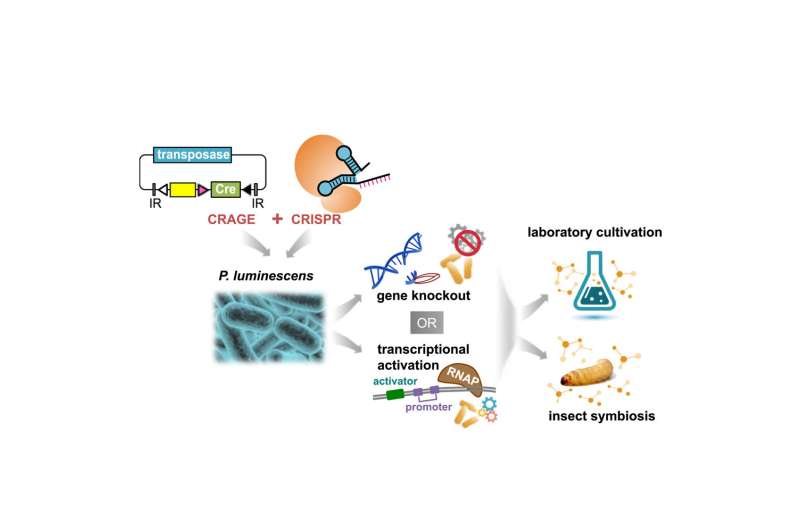
By combining CRAGE and CRISPR technologies, researchers have a much easier method for characterizing secondary metabolites. Credit: Cell Chemical Biology (2021). DOI: 10.1016/j.chembiol.2021.08.009
Microbial secondary metabolites, those molecules not essential for growth yet essential for survival, may now be easier to characterize following a proof-of-concept study in which researchers paired CRISPR and CRAGE technologies.
While CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is the leading tool for accurately editing genomes, its effectiveness has historically been limited due to the lack of robust tools available for carrying CRISPR into microorganisms. CRAGE (Chassis-independent Recombinase-Assisted Genome Engineering) is a technique researchers can use to integrate large genetic payloads directly into diverse microbes.
Combining CRAGE with CRISPR provides researchers with a powerful addition to their toolkit to study gene function. As a demonstration, the researchers used CRAGE-CRISPR for discovering active secondary metabolites and helping them identify and describe the functions of the biosynthetic gene clusters that produce them.
Secondary metabolites are microbial compounds produced in response to hardship or competition. They make up the foundation for a slew of vital products in biotechnology, medicine, agriculture, and other industries—yet there's still so much about them we don't know.
Unlocking the power of secondary metabolites can be complicated because the BGCs that produce them cannot be activated in lab environments. CRAGE made some headway in overcoming this obstacle when it hit the scene in 2019. Now, that power is poised to grow exponentially by combining it with CRISPR.
By employing CRAGE, researchers are no longer limited to using model host microbes; theoretically, any microbe can serve as the factory for producing chemical compounds of interest. Using CRAGE to domesticate target microbes, JGI users can then employ CRISPR in a variety of microbial hosts.
Photorhabdus luminescens proves fatal to insects. It is carried by an infectious nematode and releases toxins into the insect's bloodstream that quickly kill the host. Understanding exactly how P. luminescens and its secondary metabolites work could yield new tools for pest control.
Secondary metabolites are highly regulated in bacteria, making it difficult to identify which pathway corresponds to which metabolite. Finding a vehicle by which to introduce CRISPR into the microbe is key, because it allows researchers to delete or activate certain genes and assess how these edits affect functionality.
CRAGE allows for the transplanting of these BGCs from one organism into an alternate host via a landing pad that consists of a cre recombinase gene and mutually exclusive lox sites. Ultimately, this process enables researchers to identify strains able to produce secondary metabolites within a lab environment, shining a light into this "biological dark matter."
It also offers CRISPR a point of entry. By using CRISPR to knock out or activate genes, researchers at the JGI were able to monitor loss- and gain-of-function. The study's analytical data shows peaks and valleys in secondary metabolites as genes are edited. With the help of CRAGE, the pairing proved to rapidly confirm enhanced production of 22 metabolites from six biosynthetic gene clusters. One of those was a metabolite from a previously uncharacterized biosynthetic gene cluster. That work by JGI researchers was published April 2022 in Cell Chemical Biology.
When it comes to insect killer P. luminescens specifically, understanding its secondary metabolites and their pathways could fuel further agricultural applications for pest control and understanding how the pathogen uses insects for fuel.
The impact of the pairing could prove to be much more far-reaching. The compatibility of CRAGE and CRISPR could potentially allow for introducing CRISPR into other bacteria, thereby enhancing the scientific community's understanding of how secondary metabolites are produced and how to leverage their powers in agriculture, pharmaceuticals, biofuels and beyond.
More information: Jing Ke et al, CRAGE-CRISPR facilitates rapid activation of secondary metabolite biosynthetic gene clusters in bacteria, Cell Chemical Biology (2021). DOI: 10.1016/j.chembiol.2021.08.009
Journal information: Cell Chemical Biology
Provided by DOE/Joint Genome Institute