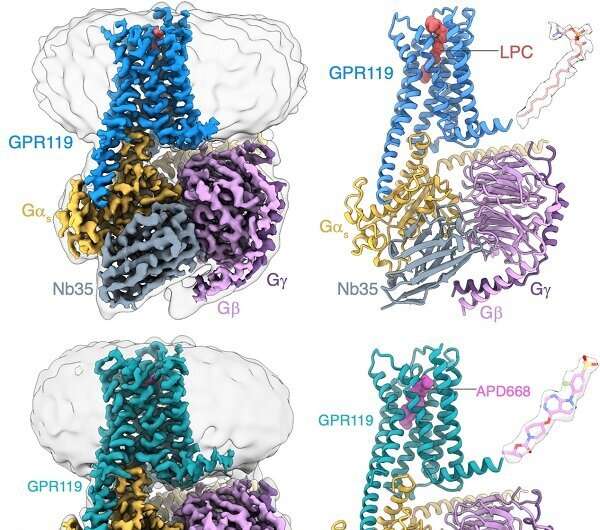
Identification of the LPC bound to GPR119–Gs complex by cryo-EM. Credit: Peiyu Xu from H. Eric Xu’s laboratory
Metabolic diseases, including diabetes, fatty liver, and obesity, have become a major "killer" affecting human health. Studies have shown that some orphan receptors could be targets for the treatment of these diseases. GPR119, also known as glucose-dependent insulinotropic receptor, is an orphan receptor in the G protein-coupled receptor (GPCR) superfamily. Activation of GPR119 can stimulate the secretion of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-releasing polypeptide (GIP), two hormones that are important for regulating the balance of glucose metabolism in the body.
GPR119 is considered as a potential drug target for the treatment of diabetes, fatty liver, obesity, and other metabolic diseases. In recent years, numerous small-molecule agonists of GPR119 have been developed by pharmacologists. However, drug discovery of GPR119 is limited by the lack of structural understanding of the recognition of ligands to the receptor.
In a study published in Nature Structural & Molecular Biology, a team of researchers led by H. Eric Xu (Xu Huaqiang) and Xie Xin from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, and Jiang Yi from the Lingang Laboratory, provided structural insights into the binding of GPR119 to the endogenous ligand lysophosphatidylcholines (LPC) and the drug candidate APD668.
Through structural biology, biochemistry, and functional studies, researchers identified that GPR119 could preferentially bind the endogenous ligand LPC. Structural analysis revealed that the ligand-binding pocket of GPR119 consisted of a hydrophobic pocket and a hydrophilic pocket. Notably, an opening was formed in the central part of the transmembrane domain, which was a unique feature from other GPCRs and provided a favorable binding space for LPC and another potential entry site for ligands.
Further studies on the structure of GPR119 in complex with APD668, a drug candidate for diabetes treatment, revealed that the rigid structural backbone of APD668 formed potent interactions with the hydrophobic pocket of GPR119. High-resolution of the structure revealed the "toggle switch," important residues to switch the activation of GPCRs. GPR119 exhibited a unique deflection mode from other GPCRs during receptor activation. A water molecule was observed to form hydrogen bonds with residues to stabilize the activated state of GPR119. In addition, the structural analysis revealed the unique recognition mode of GPR119 to the downstream signaling molecule Gs protein.
This study uncovered the molecular mechanism of the ligand-binding, receptor activation, and G protein coupling of orphan receptor GPR119, and provided structural templates and insights into the development of new drugs targeting GPR119 for the treatment of metabolic diseases.
More information: Peiyu Xu et al, Structural identification of lysophosphatidylcholines as activating ligands for orphan receptor GPR119, Nature Structural & Molecular Biology (2022). DOI: 10.1038/s41594-022-00816-5
Journal information: Nature Structural & Molecular Biology
Provided by Chinese Academy of Sciences