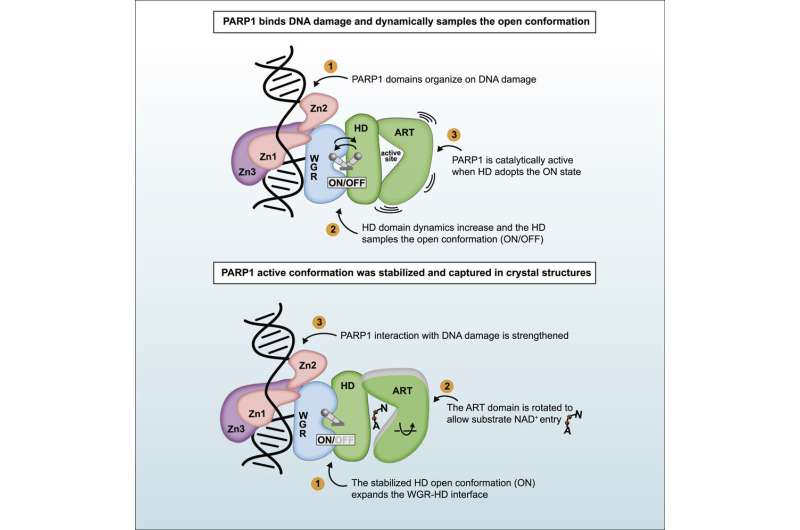
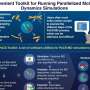
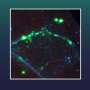
Graphical abstract. Credit: Élise Rouleau-Turcotte et al, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.06.011
New structural information about an enzyme target in cancer medicine could help the development of next generation inhibitors. The enzyme, called PARP1, senses DNA damage and sends a cellular signal to carry out repair. PARP1 activity is important to many cancer types, making it an attractive target for treatments.
Clinical studies have shown that PARP1 inhibitors can be used as anti-tumor treatments, working by disrupting DNA replication and repair to kill cancer cells. More recently, researchers have begun exploring whether PARP1 can also be used as a target in treatments for other diseases, including Alzheimer's and Parkinson's disease, where the reduction in PARP1 hyperactivity can help cells survive.
For the first time, researchers from the Université de Montréal and the Institute of Cancer Research in the UK have captured a "snapshot" of PARP1 in the active state that it adopts after detecting DNA damage. The team's new research, published in the journal Molecular Cell, furthers our understanding of how these enzymes behave and paves the way for the next-generation of PARP1 inhibitors.
The X-ray diffraction data that yielded these insights was obtained using the CMCF beamline at the Canadian Light Source (CLS) at the University of Saskatchewan and the Advanced Light Source in the U.S.
The region of PARP1 that inhibitors attack is quite mobile, making it difficult to fully understand this moving target, said Dr. John Pascal, a professor with the Department of Biochemistry and Molecular Medicine at the Université de Montréal and a member of this research team.
Certain inhibitors engage the dynamic regions of PARP1 and can act like a wrench jammed into a wheel or like a doorstop under a door, effectively helping to lock PARP1 onto DNA damage, explained Pascal. "This mode of inhibition could enhance the mechanism of killing cancer cells." In contrast, inhibitors that avoid the dynamic regions and lack the "doorstop" effect could be better suited for neurodegenerative diseases, where cell preservation is the goal rather than cell killing.
More information: Élise Rouleau-Turcotte et al, Captured snapshots of PARP1 in the active state reveal the mechanics of PARP1 allostery, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.06.011
Journal information: Molecular Cell
Provided by Canadian Light Source