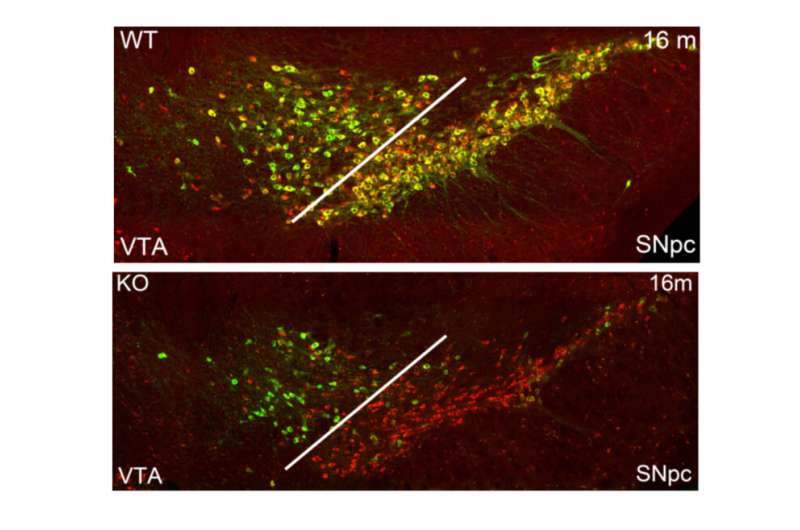
Reduced levels of TH in midbrain dopaminergic neurons of mice wherein PRC2 has been inactivated. Credit: Karolinska Institutet
A new study from researchers at Karolinska Institutet published in Science Advances show that inactivation of a protein complex that controls gene repression leads to loss of neuronal identity of dopamine-producing cells and to motor symptoms typical of Parkinson's disease.
The brain contains a large number of different neuronal subtypes which maintain their distinct cellular identities over several decades. Besides instructive information provided by transcription factors controlling cell type-specific gene programs, there is also a need to sustain silencing of transcriptional programs governing other cellular fates. The mechanisms regulating such enduring silencing in mature neurons are not well understood.
"Our work addressed the role of the Polycomb repressive complex 2 (PRC2) and its associated histone modification H3K27me3, in the maintenance of identity in differentiated dopaminergic and serotonergic neurons. Deletion of the obligate PRC2-component, Eed, in these neurons resulted in a reduction in subtype specific gene expression and a progressive impairment of dopaminergic and serotonergic neuronal function leading to behavioral deficits characteristic of Parkinson's disease and anxiety," says professor Johan Holmberg, a researcher affiliated with the Department of Cell and Molecular Biology.
"Single cell analysis revealed subtype-specific increased vulnerability to loss of PRC2 repression in dopamine neurons of the substantia nigra, the population that also predominantly affected in Parkinson's disease. Taken together, our results reveal that a PRC2-dependent non-permissive chromatin state is essential to maintain subtype identity and function of dopaminergic and serotonergic neurons."
Since both cell types are involved in several psychiatric disorders exacting a huge toll on individuals and society, it is highly relevant to attempt to understand the fundamental mechanisms governing the intact identity and function. Notably, changes in PRC2-activity and in H3K27me3 levels and distribution have been associated with neurodegenerative diseases, including Parkinson's disease and with mood disorders. Dopaminergic neurons in the substantia nigra of the midbrain are also the key population that lose function and eventually dies in PD.
The pathogenesis of this degeneration remains largely unknown. The predominantly sporadic nature of several neurodegenerative and psychiatric disorders necessitates a better understanding of how epigenetic mechanisms contribute to the etiology of pathological conditions in the CNS as well as to normal aging. Recent evidence indicates that similar mechanisms may explain the functional decline of dopamine neurons in normal aging and PD. Thus, it is possible that the process of normal aging and disease development are related and may involve loss of neuronal phenotype maintenance.
The study was carried out in transgenic mice in which the PRC2 complex had been specifically deleted in either differentiated dopaminergic or serotonergic neurons. Mutant mice were examined through histology, confocal imaging, electrophysiology, behavioral tests and global analysis of histone modification abundance and distribution through chromatin immunoprecipitation followed by sequencing (ChIP-seq) as well gene expression analysis through RNA-sequencing.
"Our study poses several questions which require further analysis. In particular, the specific vulnerability evident in the substantia nigra dopaminergic neurons is a puzzling feature. Also, the progressive loss of an additional repressive modification, H3K9me3, upon inactivation of the PRC2-complex requires further investigation. Another feature which we want to better understand is why there is a downregulation of cell type specific genes in parallel with the upregulation of derepressed PRC2 targets. Finally, we aim to investigate whether the mechanisms that we have studied in transgenic mice also are disrupted and play a role in the etiology of neurodegenerative and mental disorders."
More information: Konstantinos Toskas et al, PRC2-mediated repression is essential to maintain identity and function of differentiated dopaminergic and serotonergic neurons, Science Advances (2022). DOI: 10.1126/sciadv.abo1543
Journal information: Science Advances
Provided by Karolinska Institutet