A breakthrough for fast, efficient production of human immune cells
A University of British Columbia research team has developed a new, fast, efficient process for producing cancer-fighting immune cells in the lab. The discovery could help transform the field of immune cell therapy from an expensive, niche endeavor to something easily scalable and broadly applicable.
"We've figured out the minimal necessary steps to efficiently guide pluripotent stem cells to develop in the dish into immune cells, in particular, T cells," said Dr. Yale Michaels, referring to the most essential cells of the human immune system. "One of the next steps we're working on is to scale this up and make it work more efficiently so that we can make enough cells to treat patients."
The breakthrough paper, published last week in Science Advances by Dr. Michaels, Ph.D. student John Edgar, and a team from Dr. Peter Zandstra's lab at UBC's Michael Smith Laboratories and School of Biomedical Engineering, describes a novel method that is now the fastest known way to produce T cells in the lab.
T cells are instrumental in CAR T therapy, a well-known and successful cancer treatment that involves obtaining immune cells from the patient, genetically modifying them to fight against the patient's cancer and infusing them back into the patient's body to fight the disease. Although this type of therapy has an efficacy rate of close to 50 percent for some cancers, a new batch of medicine needs to be created for each treatment, costing roughly half a million dollars each round.
"Because the main cost associated with these treatments is the fact that they're made individually, a more cost-effective strategy could be figuring out how to manufacture those immune cells in the lab using stem cells, instead of taking them directly from a patient," explains Michaels.
Pluripotent stem cells have the ability to differentiate into any type of cell in the human body and can endlessly renew themselves. Using PSCs to create immune cells in the lab for therapeutic treatments means hundreds of doses of a medicine could be derived from a single cell.
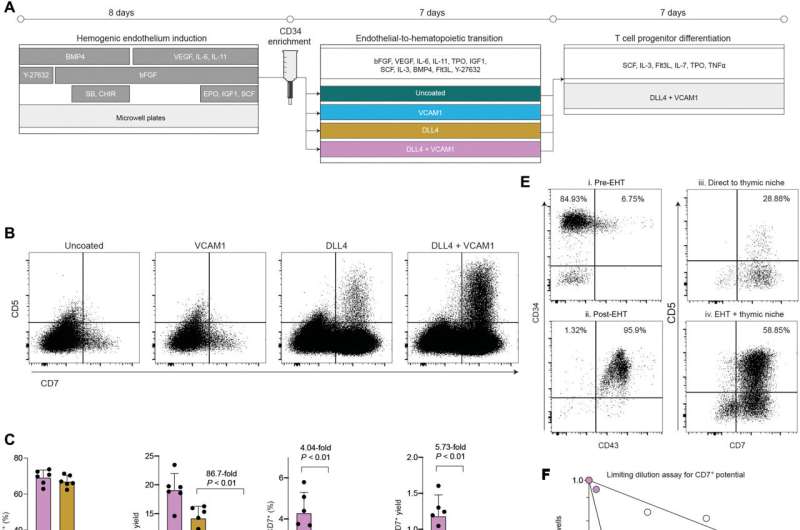
Building on a large body of previous work in the area, Michaels, Edgar and a team from the Zandstra lab discovered that providing two proteins to stem cells during a key window of development improved the efficiency of immune cell production by 80 times. By working strictly with the proteins DLL4 and VCAM1, instead of the animal cells and serums that complicated previous methods, the production process becomes a carefully controlled pipeline that is easy to replicate.
The improvement of this production pipeline is one step among many towards solving a variety of human health challenges. How to scale up a cell differentiation process, how to make cells good at killing cancer and fighting against other immune diseases, and how to deliver them to patients in a safe way are all important questions being explored simultaneously by the Zandstra lab and other research groups.
Dr. Michaels acknowledged that the collective work of thousands of people, each making important contributions, enabled this project to succeed.
"People have made tremendous progress over the last 20 years and this breakthrough is an exciting continuum," he said.
The team hopes their new findings and ongoing work in the lab will contribute to future clinical pipelines.
More information: Yale S. Michaels et al, DLL4 and VCAM1 enhance the emergence of T cell–competent hematopoietic progenitors from human pluripotent stem cells, Science Advances (2022). DOI: 10.1126/sciadv.abn5522
Journal information: Science Advances
Provided by University of British Columbia