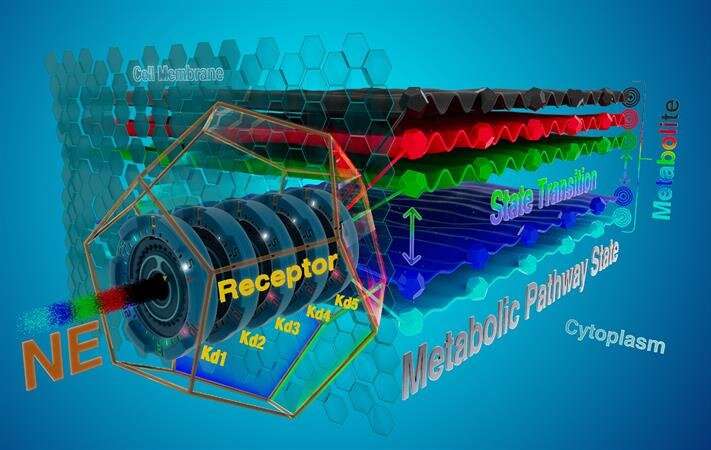
Graphic illustrates information processing in an energy metabolism pathway. Credit: EPFL Blue Brain Project
Throughout evolution, individual cells have been making successful decisions on their own, even while forming parts of vast networks, such as neurons and glia in the human brain. Now scientists from King Abdullah University of Science and Technology (KAUST) and the EPFL Blue Brain Project (École Polytechnique Fédérale de Lausanne in Switzerland) have published a new theory describing a secret language that cells may use for internal dialogue about the external world.
Using a computational model, they hypothesize that metabolic pathways, which are primarily a means of extracting energy and building block molecules from glucose and other substrates to feed the brain, might also be capable of coding details about neuromodulators that stimulate increases in energy consumption. Neuromodulators are chemical messengers that regulate the exchange of information in the brain.
If true, this implies a nearly infinite number of possibilities for information processing in nervous systems and component cell computations. Such a mechanism would also help explain the remarkable energy efficiency of brains.
The aim of the Blue Brain Project is to establish simulation neuroscience as a complementary approach to understanding the brain, alongside experimental, theoretical and clinical neuroscience, by building the world's first biologically detailed digital reconstructions and simulations of the mouse brain.
In a study recently published in the Journal of Theoretical Biology, KAUST-Blue Brain Project collaborators demonstrated how two of these pillars—theory and simulation—can work in tandem using a model of astrocytic energy metabolism. Astrocytes are star-like glial cells in the central nervous system. The model centers on how they cooperate with neurons to fuel the brain and participate in computations.
The authors confirmed the plausibility that an energy metabolic pathway might be capable of coding information and transmitting detailed characteristics about stimuli, such as intensity and duration features, in addition to its known functions in cellular energy and carbon-based molecule budgets. Examples of stimuli include waves of neuromodulators arriving at the cell surface.
Considering how many metabolic pathways are active simultaneously, these mechanisms could significantly increase the computational capabilities of neurons by giving them an expanded toolset for adaptation and decision-making. Scientists have long been impressed by the energy efficiency of the brain compared to human-made computers. Assigning new computational roles to single cells that then pass that information to neuronal networks could help explain this observation.
Co-author Pierre Magistretti, Distinguished Professor of Bioscience at KAUST and director of the KAUST Smart Health Initiative, said tht "the teams' simulations of neuromodulator-stimulated glucose metabolism in an astrocyte suggest that metabolic pathways could be capable of more information processing than we previously realized. Despite everything that is already known about how single cells think or respond to their environment, they likely still have some undiscovered tricks."
The flux of matter through these pathways involves handing-off metabolite-products from one enzyme-catalyzed reaction to the next across the entire chain of events, from neuromodulator receptor activation to energy metabolite production as an excitable unit, or metabolic state machine.
Blue Brain's Jay S. Coggan, lead author of the study, says that their "model shows how a metabolic pathway can translate external stimuli into production profiles of energy-carrying molecules such as lactate with a precision beyond simple signal transduction or amplification. Such metabolic pathways, and possibly other kinds of coupled enzymatic reactions, might be well-positioned to code an additional level of information about a cell's environmental demands. This hypothesis has implications for the computational power and energy efficiency of the brain."
More information: Jay S. Coggan et al, Representing stimulus information in an energy metabolism pathway, Journal of Theoretical Biology (2022). DOI: 10.1016/j.jtbi.2022.111090
Journal information: Journal of Theoretical Biology