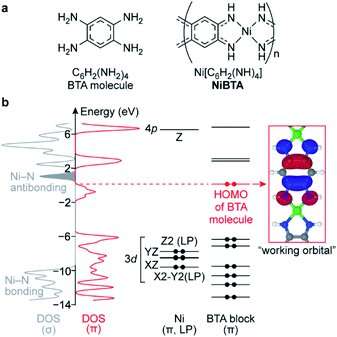
(a) Formulas of BTA molecule and NiBTA and (b) electronic structure of NiBTA. The electronic structure is partitioned into σ- and π-systems. The π-system is further decomposed into LMOs of Ni and BTA blocks. Density of Ni lone pairs (LPs) is excluded from DOS (σ). Ni LMOs are labeled by harmonic polynomials corresponding to the orbital symmetry, 3d and 4p labels are added for clarity. Electrons in BTA and Ni diagrams are shown as dots. The red dots correspond to electrons of BTA that are absent in the ligand moieties of NiBTA. 3dXY, 4s, 4pX and 4pY orbitals of Ni are highly intermixed with other orbitals into σ-bands and are not shown here. Credit: Chemical Science (2022). DOI: 10.1039/D2SC03127B
Skoltech researchers with the help of their colleagues from Moscow State University unveiled the charge storage mechanisms of NiBTA—a recently discovered material that can enable advanced fast-charging batteries. Their report is published in Chemical Science.
Fast-charging batteries are needed for many applications, such as electric vehicles that suffer from the "range anxiety" issue. However, modern battery anode materials are either unsuitable for reliable fast charging or afford only moderate energy density. It motivates the scientists to search for new materials that have larger capacities and can safely operate under fast-charging conditions.
Recently, researchers proposed NiBTA, a new promising anode material which is a nickel-based coordination polymer derived from benzenetetramine. However, it remained unclear how NiBTA is charged and discharged in batteries. Different research groups proposed entirely different mechanisms, mostly because the data were too ambiguous to give reliable conclusions. In the new work, Skoltech scientists used a combination of advanced methods to gain insights about NiBTA behavior in lithium-, sodium-, and potassium-based batteries.
"The beauty of this work is putting together various techniques, both experimental and theoretical," the first author of the study, Skoltech Professor Roman Kapaev said. "This helped to get trustworthy results because each method gives only a part of the picture. Among other things, we used operando X-ray diffraction and operando Raman spectroscopy, which allowed us to track the structural changes inside the batteries in detail. We were the first to apply such a rigorous approach for this class of compounds. This study sheds light on the redox chemistry of coordination polymers, which can be useful for many applications."
More information: Roman R. Kapaev et al, Charge storage mechanisms of a π–d conjugated polymer for advanced alkali-ion battery anodes, Chemical Science (2022). DOI: 10.1039/D2SC03127B
Journal information: Chemical Science
Provided by Skolkovo Institute of Science and Technology