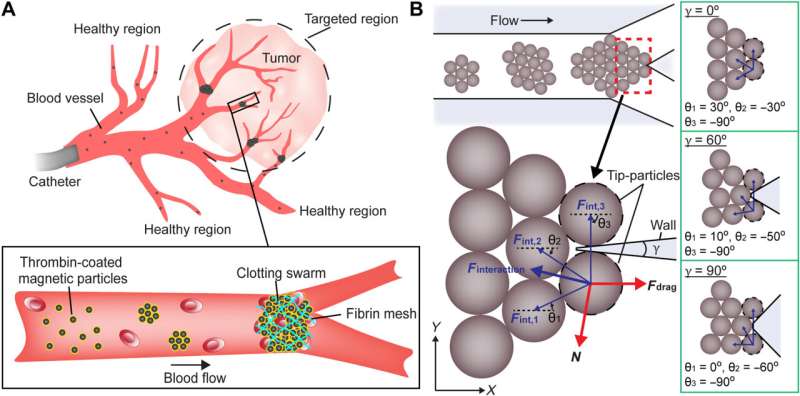
Maintenance of swarm integrity at targeted junctions. (A) Schematics illustrating the use of magnetic particle swarms to block the junctions inside a targeted region. (B) Schematic analysis of the forces exerted on tip-particles. The brown circles indicate magnetic particles. The black dashed circles denote the tip-particles. The magnetic interaction forces and their resultant interaction force are indicated by thin blue arrows and a thick blue arrow, respectively. The fluidic drag force and the reaction force are indicated by thick red arrows. γ is the branching angle of the junction. θ is the angle between the magnetic interaction force and the x axis. The configurations of particles at junctions with different branching angles are demonstrated in the green boxes. Purple regions represent the walls of junctions. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm5752
Microrobotic agents can form swarms of targeted drug delivery for improved imaging analyses. In a new report now published in Science Advances, Junhui Law and a team of researchers in mechanical and industrial engineering, artificial intelligence and biomedical engineering at the University of Toronto and the Shanghai University, China, deviated from the typical process of drug therapy to facilitate swarm embolization. The process is a medical technique used to block blood vessels during treatment for thrombosis and arteriovenous malformations. Magnetic particle swarms offer more precise embolization and can maintain swarm integrity inside a targeted region under fluidic flow conditions. Based on experiments in microfluidic channels, ex vivo tissues and in vivo porcine kidneys, Law and the team validated the efficacy of the proposed strategy for selective embolization.
Collective swarms
Collective behaviors are ubiquitous in nature, where schools of fish and swarms of insects can perform complex tasks. Bioengineers are inspired by the collective intelligence in natural swarms to develop a variety of microrobots for diverse applications. In this work, the researchers developed an actuation strategy to integrate magnetic particle swarms to accurately embolize blood flow inside a targeted region for selective embolization in an animal model. The work provided deeper insight and a proof-of-concept study to understand micro-robotic swarm behavior under physiological conditions.
Swarm integrity during flow
The research team achieved selective embolization by generating microrobotic swarms on demand to block blood vessels within a targeted region. They used super-paramagnetic particles with diameters smaller than red and white blood cells for their distribution in blood capillaries. The researchers coated the microparticles in thrombin to convert soluble fibrinogen in blood into fibrin meshes to contain red blood cells with the particles.
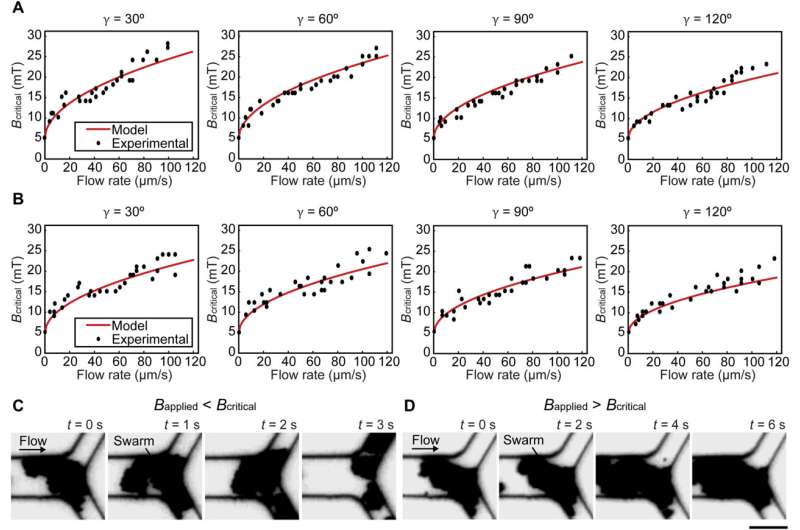
The team noted how the swarms split under flow due to weak interactions between the particles. The research team sustained swarm integrity within microfluidic channels under physiologically relevant conditions, including blood vessel branching and blood flow. They then modeled a swarm at a junction to understand the relationships between the branching angle, flow rate and swarm integrity relative to magnetic field strength. While swarms split when the applied magnetic field strength was lower than the calculated value, swarms maintained their integrity at a junction when the applied magnetic field strength was higher than the calculated value.
Experimental validations for the model. (A and B) The relationship between critical magnetic field strength Bcritical and flow rate at junctions with different branching angles γ in porcine whole blood and PBS, respectively. (C and D) The integrity of swarms when the magnetic field strength applied was lower and higher than Bcritical, respectively. Scale bar, 20 μm. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm5752
Selective maintenance of swarm integrity
The scientists sought to develop low magnetic field strength for selective embolization to degrade the integrity of swarms and prevent unintended blockage. They maintained an actuation strategy for sustained swarm integrity inside a targeted region. Despite changing magnetic field distributions, the team maintained high magnetic field strength within the targeted region. Swarms that formed outside the target region encountered low-strength magnetic fields and could not therefore maintain their integrity. The scientists validated the proposed actuation strategy via experiments.
Embolization in microfluidic channels and proof-of-concept studies
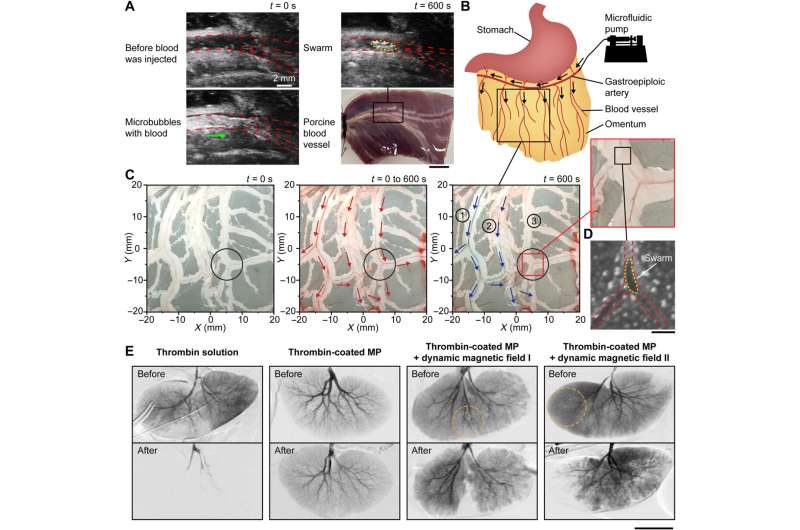
The research team tested the effectiveness of using magnetic particle swarms to block blood flow and measured the blood flow rate under different conditions. They ensured visibility under optical microscopy by diluting porcine blood flow in microfluidic channels with 1200 branching angles. The team measured the flow rate by calculating the speed of the red blood cells to understand the average flow rate, which amounted to an average of 84 µm/s. The scientists demonstrated an actuation strategy together with thrombin-coated magnetic particles for selective embolization with minimal unintended blockage beyond a target region. They then conducted proof-of-concept experiments in a porcine blood vessel ex-vivo using microrobotic swarms and imaged a blood vessel with a branching angle of 30 degrees via an ultrasound imaging system. They additionally injected thrombin-coated magnetic particles into the blood vessel at a flow rate of 80 µm/s and noted a brightened spot at the junction indicating the formation of a swarm to confirm the embolization of the blood vessel via the swarm. After ex vivo studies, the team tested the proposed strategy for selective embolization in in vivo porcine kidneys to realize selective embolization.
-
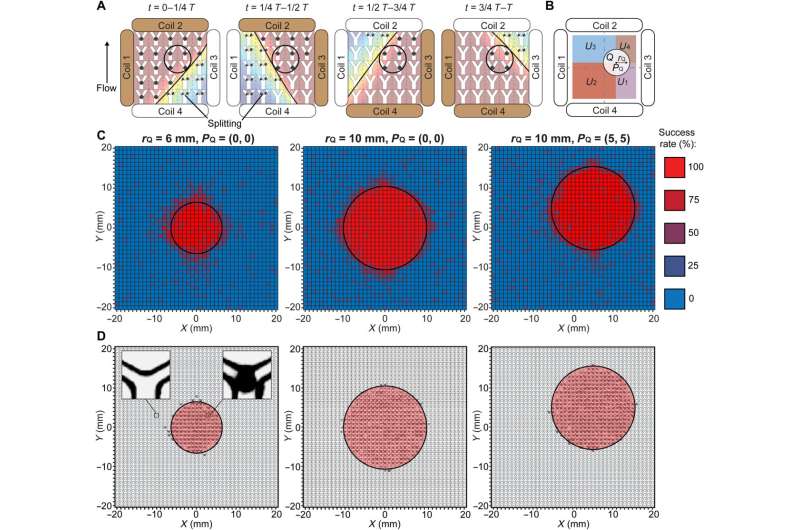
Actuation strategy for selective maintenance of swarm integrity and experimental validation. (A) Schematic illustration of the proposed actuation strategy. The black circles indicate the targeted region. The brown and white coils are the dominant and auxiliary coils, respectively. The black lines separate the workspace into regions with the magnetic field strengths higher and lower than Bcritical. The black arrowhead denotes the flow direction. (B) Schematic illustration of the targeted and nontargeted regions described in the brute-force search. The black circle indicates the targeted region. The radius rQ and the center position PQ of the targeted region are labeled. The nontargeted subregions U1, U2, U3, and U4 are highlighted with different colors. (C) Experimental success rate of the proposed strategy in maintaining the swarm integrity in three cases. The experimental data in each small square were measured from independent microfluidic channels, and four experiments were repeated to determine the success rate. The black circles indicate the targeted regions. (D) Experimental spatial distribution of locations with a success rate of 75% and above in three cases. The left inset shows an empty junction indicating that swarms were split, and the right inset shows a swarm successfully maintained at a junction. The black circles indicate the targeted regions. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm5752
-
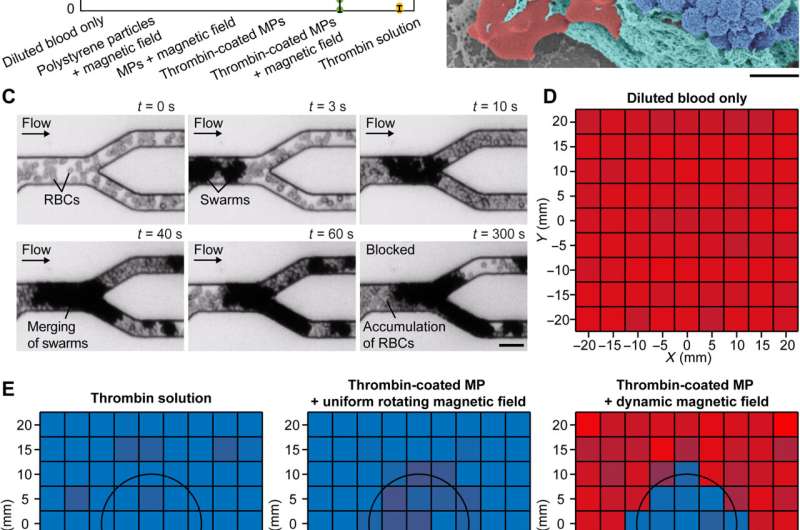
Embolization in microfluidic channels. (A) Different conditions for reducing blood flow rate. The flow rates were measured when the conditions were kept activated for 10 min. The error bars represent the SD of 10 trials. MPs denote magnetic particles. (B) Scanning electron microscopy image of a clotting swarm. For visualization, porcine RBCs, fibrin meshes, and magnetic particles were artificially colored in red, green, and blue, respectively. Scale bar, 2 μm. (C) Experimental results of embolization in microchannels using thrombin-coated magnetic particles. Scale bar, 20 μm. (D) The input flow rate of diluted porcine blood in the microfluidic channels (average flow rate: 83 μm/s). (E) Experimentally measured flow rate in the microfluidic channels under different embolization conditions. The flow rates were measured when the conditions were kept activated for 10 min. For (D) and (E), the data in each small square were measured from independent microfluidic channels, and three experiments were conducted to obtain an average flow rate. The black circles indicate the targeted region. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm5752
-
Embolization in porcine organs. (A) Formation of a clotting swarm at the junction of an ex vivo porcine blood vessel. The red dashed lines outline the blood vessel and junction, and the yellow dashed line outlines the clotting swarm. The green arrow shows the flow direction of microbubbles. Scale bar, 10 mm. (B) Schematic illustrating the injection site of an ex vivo porcine omentum in experiments. Black arrows indicate the flow direction. (C) Selective embolization in the blood vessel network of an ex vivo porcine omentum with the targeted region centered at (5 mm, −5 mm). The black circles indicate the targeted region, the red arrows indicate the blood flow direction, and the blue arrows indicate the flow direction of blue dye. (D) Optical microscopy image showing a swarm formed at the targeted junction of an ex vivo porcine omentum. The red dashed lines outline the blood vessel and junction, and the yellow dashed lines outline the magnetic particle swarm. Scale bar, 200 μm. (E) Digital subtraction angiography results of in vivo porcine kidneys under different embolization conditions. The orange dotted circles indicate the targeted regions. Scale bar, 50 mm. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abm5752
Outlook
In this way, Junhui Law and colleagues developed an actuation strategy to regulate magnetic particle swarms for selective embolization. The microrobotic swarms formed via the actuation strategy provide a potential solution for selective embolization in the clinic to prevent complications arising via non-selective embolization mechanisms.
More information: Junhui Law et al, Microrobotic swarms for selective embolization, Science Advances (2022). DOI: 10.1126/sciadv.abm5752
Changjin Wu et al, Ion-exchange enabled synthetic swarm, Nature Nanotechnology (2021). DOI: 10.1038/s41565-020-00825-9
Journal information: Nature Nanotechnology , Science Advances
© 2022 Science X Network