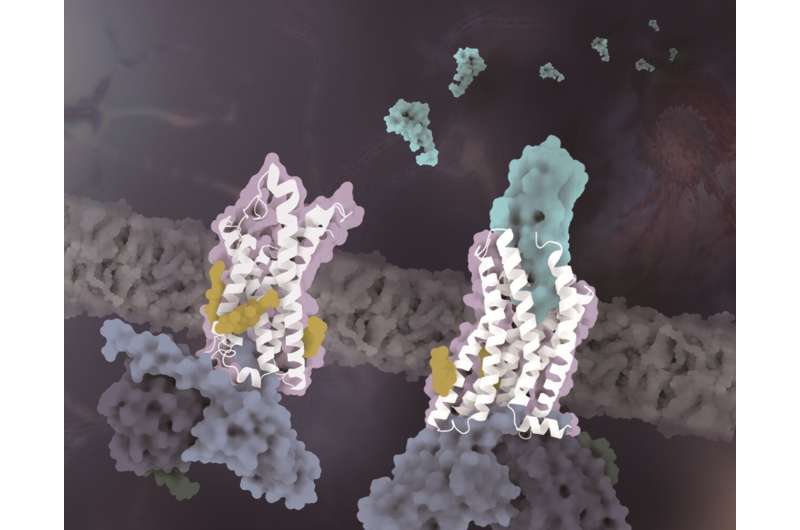
The structures of CX3CR1 in distinct conformational states. The CX3CR1 in both ligand-free state and CX3CL1-bound state are colored white. The CX3CL1 is colored cyan, the cholesterols are colored yellow and the three subunits of the G protein are colored light blue, dark blue and dark green, respectively. Credit: Zhao Qiang's laboratory at SIMM
Chemokine receptors regulate the migration of immune cells and are involved in inflammation, tumor construction and pathogen infection. Chemokines are divided into four subfamilies according to the number and distribution of conserved cysteines at the N terminus: CC, CXC, CX3C, and XC.
The chemokines recognize their receptors in the same subfamily which complicate targeted drug development. As the only member of the CX3C chemokine receptor subfamily, CX3CR1 presents unique advantage as a potential drug target in the treatment of atherosclerosis, cancer, and neuropathy. However, the drug development of CX3CR1 is hampered partially by the lack of structural information that governs chemokine recognition and receptor activation.
In a study published in Science Advances on June 29, a research team led by Zhao Qiang and Wu Beili of the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, made a breakthrough in the field of chemokine receptors by solving the cryo-electron microscopy structures of CX3CR1-Gi and CX3CR1-CX3CL1-Gi complexes.
Although several chemokine receptor complex structures in the CC and CXC subfamilies have been solved, the molecular mechanism of the unique recognition of CX3CR1 and CX3CL1 remains unknown. With the analysis of structures and sequence alignment, the researchers found that the unique structural features of the 30s loop in CX3CL1 and the ECL2 region in CX3CR1 play a key role in the recognition with shape complementarity mechanism. Compared with the CC and CXC chemokines, the specific CX3C motif of CX3CL1 results in a larger shift of the 30s loop towards the CX3CR1 ECL2.
As the ECL2 of CX3CR1 contains fewer residues than other chemokine receptors, correspondingly, the shorter ECL2 in the CX3CR1-CX3CL1 structure provides sufficient space for the 30s loop of CX3CL1. However, the 30s loop of other chemokine subfamilies exhibits unextended conformation, which is complementary to the surface of longer ECL2 of the corresponding receptors.
This is the first time scientists have provided the structural basis for elucidating the molecular mechanism of specific recognition between CX3CR1 and its unique endogenous ligand.
In addition to the specificity of chemotactic signal recognition, another important finding of this study reveals that cholesterol molecules regulate the activation of CX3CR1. In the complex structures of both CX3CL1-bound and constitutively activated states, three cholesterols were observed to stabilize the helix VI of CX3CR1 with a much smaller conformational change than previously solved class A GPCR-Gi complex structures. Supported by functional data of CX3CR1 and other chemokine receptors, the cholesterols are further verified to play specifically essential roles in conformation stabilization and signaling transduction of CX3CR1.
This study provides insights into the unique chemokine recognition mechanism for the human chemokine receptor subfamily. The distinct cholesterol-binding sites of CX3CR1 deepen our knowledge about the modulation of cholesterols in GPCRs.
More information: Minmin Lu et al, Activation of the human chemokine receptor CX3CR1 regulated by cholesterol, Science Advances (2022). DOI: 10.1126/sciadv.abn8048
Journal information: Science Advances
Provided by Chinese Academy of Sciences