Caught in the act: Key chemical intermediates in pollutant-to-fuel reaction identified
Carbon dioxide pollution continues to change the global climate. Researchers know how to pinpoint such pollution, even on a regional and near-real-time basis. As part of a solution to carbon dioxide pollution, many studies focus on how to convert this pollutant into a fuel, such as methanol. Copper-based catalysts are a tool for such conversions. Understanding the corresponding step-by-step chemistry is essential for optimizing conversion of carbon dioxide pollutant into methanol fuel. However, the details of this chemistry remain unclear; experiments are needed to test hypotheses that are currently based on computer simulations.
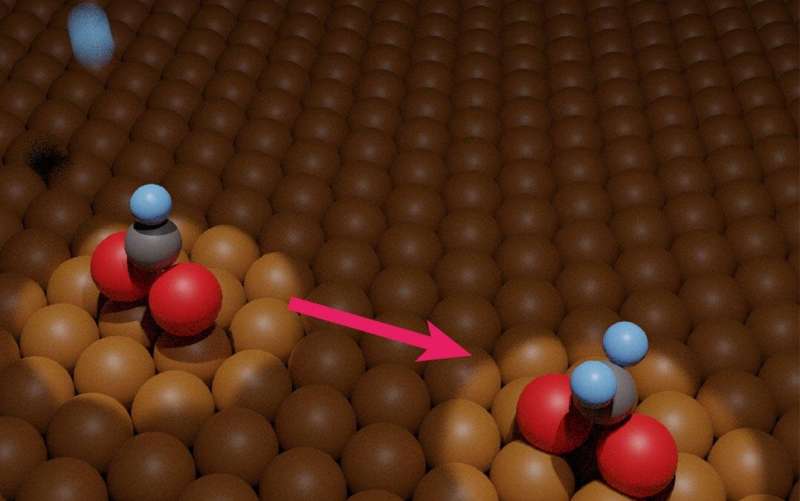
Now, in a study recently published in Journal of the American Chemical Society researchers from the University of Tsukuba and collaborating partners have experimentally measured hydrogenation of copper-adsorbed formate. This study will help researchers optimize critical steps in the aforementioned pollutant-to-fuel process, and thus accelerate methanol production.
"Hydrogenation of carbon dioxide into methanol is a potential key technology for producing fuel and chemical feedstocks, but optimizing the reaction remains difficult," explains Dr. Kotaro Takeyasu, senior author. "That's because it's difficult to experimentally detect chemical intermediates in the step-by-step reaction mechanism."
Infrared reflection absorption spectroscopy and temperature-programmed desorption were critical to obtaining two main findings. First, at a temperature of 200 Kelvin, exposure to atomic hydrogen corresponded to hydrogenation of adsorbed formate. The exact chemical nature of the product isn't yet clear. It was also found that, at a temperature of 250 Kelvin, the hydrogenated formate decomposed back into adsorbed formate or gaseous formaldehyde, in a 96:4 ratio.
"On the basis of our experimental and computational work, the activation energy of the hydrogenation of adsorbed formate is approximately 121 kilojoules per mole," states Dr. Takeyasu. "Our results are consistent with reported results of methanol synthesis studies."
Copper-zinc alloys are particularly common in this line of work. The research group is currently investigating how the activation energies reported in the present study compare with particularly useful catalytic alloys, which also require experimental and computational investigations.
The results of this study will help researchers optimize methanol production from carbon dioxide. Such work will help convert an atmospheric pollutant into fuel for vehicles, and chemical feedstocks for industry. It provides a means of adding value to carbon dioxide, which is commonly considered to be waste. By optimizing the hydrogenation reaction described here, researchers might have a new tool for making maximum use of limited resources.
More information: Kotaro Takeyasu et al, Hydrogenation of Formate Species Using Atomic Hydrogen on a Cu(111) Model Catalyst, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c02797
Journal information: Journal of the American Chemical Society
Provided by University of Tsukuba