New catalysts make efficient use of precious metals
Nanoscientists from Utrecht University have devised a new and promising way to make catalysts in which the amount of precious metals needed is reduced by a factor of 10. Those precious metals are scarce, but essential in many existing and future sustainable chemical processes. The publication appeared on 8 July in Science.
Precious metals such as platinum are widely used in industry and in daily life. The best known application is currently in the exhaust gas catalyst of cars to clean the combustion gases of the engine. But precious metals will also be needed in the future to achieve a more sustainable society, for example for the production and consumption of hydrogen, an important energy carrier of the future.
But the amount of precious metals in the world is very limited, so it is a major challenge to reduce the quantities needed. Ph.D. researcher Luc Smulders of the Debye Institute for Nanomaterials Science at Utrecht University says that "the world stock of platinum is estimated at 70,000 tons, which is about 10 grams per world inhabitant. One fuel cell in a car, to produce electrical power from hydrogen for the electric motor, already requires about 10 grams of platinum. This gives a sense of the need to use platinum as effectively as possible."
Applying platinum to zeolite with precision
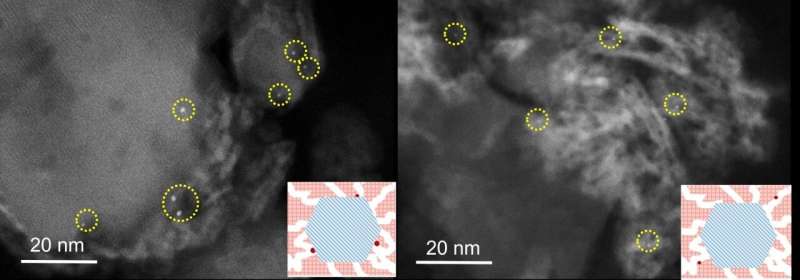
Together with a former postdoctoral fellow at the same institute, Kang Cheng, Smulders, under the supervision of Emeritus Professor Krijn de Jong, investigated how platinum can be used in catalysts as effectively as possible. De Jong says that "the catalysts in this study contain two active functions, namely a metal—platinum—and an acid function—a zeolite. Classically, the precious metal is saved by making the platinum particles as small as possible. Such small particles, also called nanoparticles, have a greater ratio of surface area per unit volume."
The Utrecht chemists have now followed a completely different path. Using special synthesis techniques, they positioned the platinum particles with precision in relation to other active material present in the catalyst. Smulders says that "usually the nanoparticles in catalysts are distributed randomly over the material. We discovered that the catalytic effect of platinum is just as good—and much less of it is needed—if it is only applied to the surface of the zeolite crystals, instead of inside or next to the zeolite."
On an industrial scale within two years
De Jong says that "this means that only a tenth of the amount of platinum is needed without affecting the performance of the catalyst." The work is therefore a breakthrough for using precious metals many times more efficiently in catalysts, and possibly also in other applications that are essential for achieving a more sustainable society. The researchers expect that it will be possible to use the technique on an industrial scale in existing processes within one to two years.
More information: Kang Cheng et al, Maximizing noble metal utilization in solid catalysts by control of nanoparticle location, Science (2022). DOI: 10.1126/science.abn8289
Journal information: Science
Provided by Utrecht University Faculty of Science




















