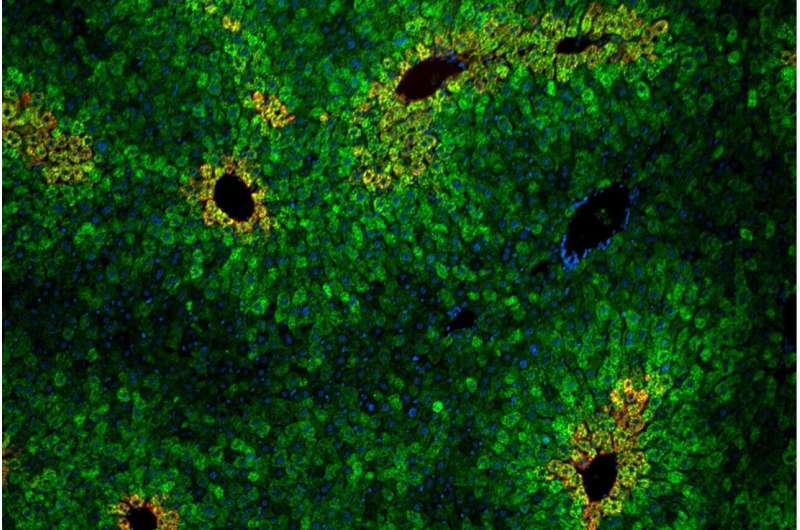
Cells in the liver are arranged in “lobules” of concentric rings of hepatocytes. Liver cells within the lobules vary in their sensitivity to the essential amino acid leucine. Credit Andrew Cangelosi/Whitehead Institute
A new paper from Whitehead Institute researchers reveals how mice sense an essential amino acid called leucine, which many people get from eating fish, eggs, or nuts. Down the line, the work could inform research into creating drugs that target specific parts of a key metabolic and growth-regulating pathway called the mTOR pathway to treat some cancers or other metabolic diseases.
Leucine is necessary to build and repair muscle in the body; if the body cannot access this amino acid from food, its best course of action is to shut off metabolism in certain tissues until the resource is restored. That's why leucine sensing is important—if the animal's metabolism keeps running as usual sans leucine, the researchers found that the animal will essentially cannibalize itself, depleting fat and muscle stores.
Former Whitehead Institute researcher Andrew Cangelosi led the study while completing his Ph.D. in the lab of former Whitehead Institute Member David Sabatini. "People have known for a long time that amino acids very strongly regulate the mTOR pathway, but when I started in the lab, it was a big black box—we were just beginning to understand what they were doing and how that was happening," Cangelosi said.
Over the past 15 years, researchers—at Whitehead Institute and elsewhere—have teased apart some of the mechanisms of how amino acids affect the pathway. "One of the big insights that came from this was that the pathway cared about very specific amino acids," Cangelosi said. There are 20 amino acids used by mammalian cells to create proteins, and a handful of these—including leucine—have a much stronger effect on the mTOR pathway than other amino acids.
In a 2014 paper, researchers at Whitehead Institute discovered that a family of proteins called the Sestrins were responsible for communicating the presence of leucine to the mTOR pathway, specifically mTORC1, the nutrient sensing complex. (The protein mTOR is an essential component in two different protein complexes, mTORC1 and mTORC2, which play different roles in the body. mTORC1, is sensitive to nutrients and controls protein synthesis and cell growth in response, while mTORC2 is involved in cellular signaling and metabolic regulation.) In cultured cells, Sestrin1 and Sestrin2 inhibit mTORC1 signaling by interacting with and suppressing a protein complex called GATOR2. When GATOR2 is suppressed, the mTOR pathway cannot stay active.
This research took place in cell culture, however, so questions remained as to how this mechanism was playing out in live mice. "Studying a homogenous cell population in a dish is very different from in an animal," he said. "We really wanted to understand what the results in cell culture meant for what leucine does in the body."
Cangelosi spent his graduate years developing mouse models without Sestrins in order to test whether the proteins were playing the same role in animal models that they were in a dish. He then fed these mice, as well as control mice, a diet completely free of leucine. When normal mice were deprived of leucine, they were able to compensate by turning off the mTOR pathway and slowing/halting metabolism. But when mice without Sestrins (and therefore, the ability to sense leucine) were fed the leucine-free diet, they drastically lost fat and muscle mass.
As in cell culture, the leucine-sensing pathway depended on the protein complex GATOR2, and was specific to mTORC1 (not mTORC2). One new insight from the animal models was that leucine sensing was concentrated in specific areas of the liver. These zones, called liver lobules, are hexagonal arrangements of cells that direct the nutrient-rich blood from the gut through the liver's filtering system and into the body's circulation.
"The liver essentially sees anything you eat before the rest of your body does," Cangelosi said. "It acts as a sort of nutrient gateway in the body, and different cells in the liver have different properties depending on their arrangement. It definitely seems like the body is leveraging the Sestrins to make the mTOR pathway sensitive or not sensitive to leucine depending on where it needs to be or not."
The insight that even within the liver, not all cells respond the same way to leucine presence or absence suggests a more complex view of this metabolic process, Cangelosi said. "This points to a very new way in which the entire pathway works in the body—that it's wired differently in different contexts in different settings, so that cellular metabolic function can be actually dictated by the specific environment of the cell or tissue."
Although some medicines are designed to induce fat loss, Cangelosi stressed that the fat loss seen in the leucine-insensitive mice is not healthy. "I don't think it could be considered beneficial," he said. "The mTOR pathway is a process of nutrient preservation that is important, and the mice also lost a lot of muscle mass as well. This isn't some healthy metabolic reprogramming—it was a pretty bad response for the mice."
Cangelosi's research could inform therapies in other ways, however. Understanding how mTOR-related processes play out differently in various cell types could eventually lead to therapies for some cancers and other diseases that affect cell metabolism. Currently mTOR targeting drugs—specifically those based on the immunosuppressive drug rapamycin—often stall in clinical trials because of a lack of specificity.
"When [mTOR-targeting drugs] are given to people, the issue always comes down to how they just so broadly just shut off everything that mTOR does," Cangelosi said. "If we have a better understanding of how we can specifically target distinct mTOR complexes—and, this may be a long shot, but if we can identify ways of targeting in specific regions of the body, even specific cells of the body—that are important for the given disease or pathology that we're trying to treat, these would be critical to getting rid of the negative side effects that have been limiting this in the clinic for so long."
The research was published in Science.
More information: Andrew L. Cangelosi et al, Zonated leucine sensing by Sestrin-mTORC1 in the liver controls the response to dietary leucine, Science (2022). DOI: 10.1126/science.abi9547
Journal information: Science
Provided by Whitehead Institute for Biomedical Research