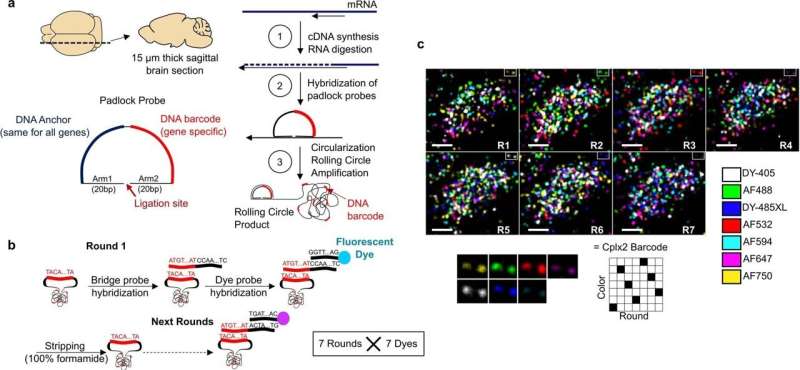
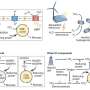
Detection of 72 genes using coppaFISH. a, Sagittal 15 µm brain sections are cut using a cryostat. Local mRNAs are reverse transcribed to cDNA, and the mRNAs digested to free the cDNAs for hybridization with padlock probes. Padlock probes have two 15-20-nt arms complementary to the target site, a 20-nt anchor sequence (identical for all probes) and a 20-nt barcode sequence (unique for each gene). After hybridization to the target site, a DNA ligase enzyme circularizes the padlock probe, but only when it matches the target perfectly. Next, a DNA polymerase enzyme amplifies the circularized padlock probes, producing rolling-circle products (RCPs), which contain many repeats of the padlock sequence including the barcode. b, The barcodes are read out by 7 rounds of 7-color fluorescence imaging. On each round, RCPs are hybridized with custom designed bridge probes, which in turn hybridize to specific dye probes (conjugated to one of 7 fluorophores). The sections are then imaged in 7 color channels, then all DNA is removed with formamide treatment, and the next round begins. Different sets of bridge probes on each round result in each barcode showing up in a different color channel using a Reed–Solomon code for minimum overlap. After the 7 combinatorial rounds, a final round images the anchor probe (used for image alignment) and DAPI to visualize cell nuclei. c, Example raw data for one cell imaged with the 7 fluorophores and 7 rounds. Each fluorescent spot is an RCP, and the sequence of colors across 7 rounds allows gene identity to be determined. Bottom: magnification of 2 RCPs (top right corner of main images) which corresponded to Cplx2 barcode (6135024). Scale bars: 5 µm. Credit: Nature (2022). DOI: 10.1038/s41586-022-04915-7
A team of researchers from University College London, Columbia University and Oxford University has developed a new approach to conducting transcriptomics and has used it to reveal the properties of 35 neuron subtypes in mice. The group has published their research in the journal Nature. Hongkui Zeng and Saskia E. J. de Vries with the Allen Institute for Brain Sciences have published a News & Views piece in the same journal issue outlining the work done by the team.
One of the goals of biological scientists is to learn how the brain works, in other animals and in humans. Considering the complexity of the brain, the goal is ambitious to be sure. But scientists believe that it can be done by carrying out a variety of step-by-step studies that seek to explain the various parts of a given brain to describe what they do. Part of that effort involves creating a catalog of brain cell types and subtypes.
One aspect of cell type classification involves deciphering and recording the repertoire of expressed genes—a process called transcriptomics. From this comes the identification of the roles that each of the individual genes play in brain circuitry. To that end, researchers have used various techniques to conduct transcriptomics (which have to be carried out on live tissue) to learn more about regions of the brain, such as the mouse visual cortex. To date 95 groups have been identified.
In this new effort, the researchers have developed a new way to carry out such work—they call it coppaFISH, and it is capable of determining the expression of 72 genes at once from a thin slice of brain tissue. It involves combining in vivo two-photon calcium imaging with conventional transcriptomic methods. The resulting expression profiles for the genes can then be used to map the transcriptomes to cell identities.
The researchers used the technique to obtain new data for 1,090 interneurons, involving 35 neuron subtypes. In so doing they found that certain transcriptomic principles could be used to predict the activity of other cell types, resulting in a transcriptomic axis that correlates with some types of cell properties. The researchers suggest their technique could be used in other regions of the brain as well.
More information: Stéphane Bugeon et al, A transcriptomic axis predicts state modulation of cortical interneurons, Nature (2022). DOI: 10.1038/s41586-022-04915-7
Hongkui Zeng et al, A gene-expression axis defines neuron behaviour, Nature (2022). DOI: 10.1038/d41586-022-01640-z
Journal information: Nature
© 2022 Science X Network