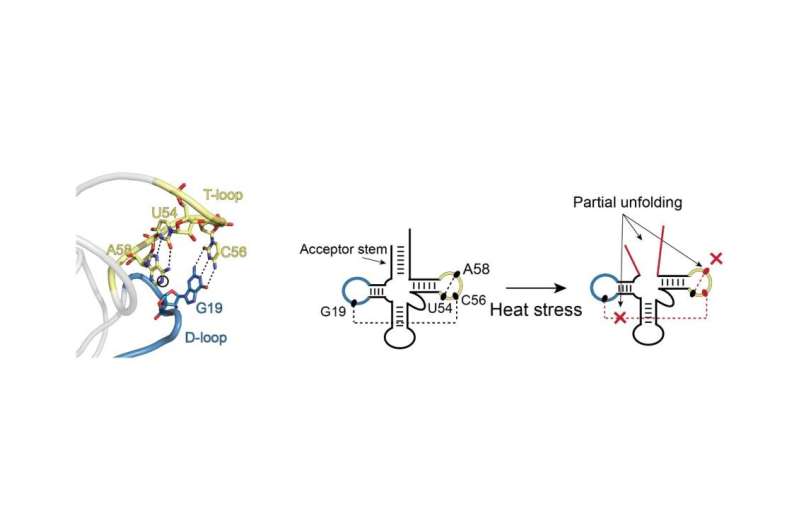
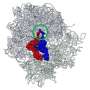
An example of partial unfolding of tRNA under heat stress. The image on the left shows the natural three-dimensional conformation of a portion of the tRNA with the D-loop (blue) and T-loop (yellow) connected in what is referred to as “kissing loops.” The two images on the right show the transition in structure that occurs upon heat stress. D- and T-loops retain their blue and yellow coloring. There is partial unfolding of the acceptor stem and the kissing loops no longer kiss. Credit: Bevilacqua Lab, Penn State
A new method allows researchers to determine the structure and abundance of "transfer RNAs" (tRNA)—small, highly structured and chemically modified RNAs involved in protein production—in living cells. Misfolding of tRNAs has been linked to human diseases ranging from cancer to type 2 diabetes and neurological disorders. The new method, which also shows how tRNA structure can change when the cells are stressed by high temperatures, a common stressor faced by plants, bacteria, and even humans, could potentially inform the development of therapeutics for RNA-linked diseases and is applicable to other small, highly modified types of RNA.
A paper describing the method, developed by researchers at Penn State, appears this week in the journal, Proceedings of the National Academy of Sciences.
"About eight years ago, our team developed a method called 'Structure-seq' that uses chemical modifications and high-throughput sequencing to determine the structure of messenger RNAs in vivo," said Philip Bevilacqua, distinguished professor of chemistry and of biochemistry and molecular biology at Penn State and a leader of the research team. "We've expanded that method here to overcome the challenge of working with smaller RNAs that have very stable structures and are naturally highly modified with chemical tags, like tRNA."
RNA is similar in many ways to DNA. Both are long molecules composed of small units called "bases" or "nucleotides," represented by A, T, C, and G in DNA (the T is replaced by U in RNA). But where DNA is double-stranded—two long molecules that run alongside each other connected by hydrogen bonds between complementary units—RNA is just a single strand. This difference is essential as RNA molecules get their structure by folding back on themselves forming short sections where the units bond like double-stranded DNA but interspersed with single-stranded segments that form loops and bulges.
The general structure of tRNA was determined in the 1960s and 70s and is classically described as a cloverleaf with three loops connected by double-stranded segments around a central hub. There are dozens of types of tRNA that vary only slightly in their sequence. Each type of tRNA corresponds to a specific amino acid—the building block units of proteins—and their structures are vital to their function in delivering the amino acids to the site of protein production in the cell.
"We can predict the structure of RNA molecules based on their sequence and by modifying the molecules with the chemical dimethyl sulfate (DMS), which adds a different chemical tag to certain nucleotides that are exposed in the structure," said Ryota Yamagami, a postdoctoral researcher at Penn State at the time of the research and now an assistant professor at Ehime University in Japan. "Because tRNAs are small and already highly modified, which can cause problems for sequencing, we had to develop methods to ensure that we captured the full-length molecules."
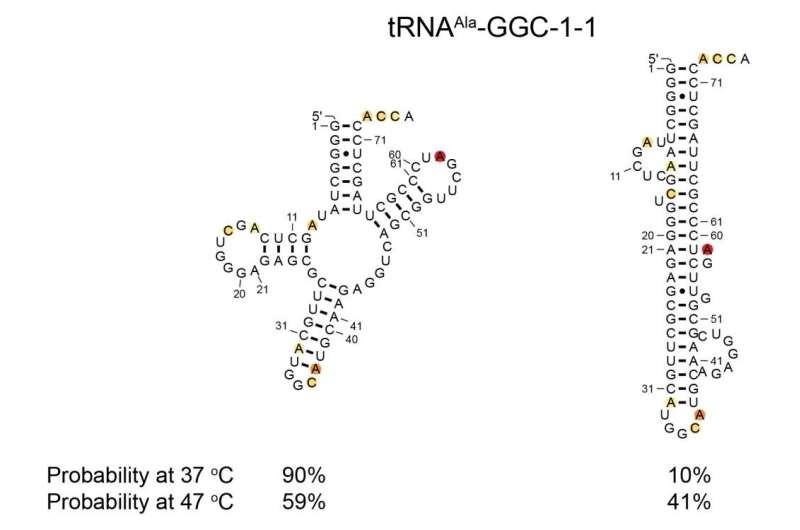
Change in tRNA structure abundance due to heat stress. The image shows two potential configurations of a specific tRNA molecule. The image on the left shows the natural, functional cloverleaf structure, the image on the right shows a misfolded rod-like configuration. When grown under natural conditions at 37 degrees Celsius, only 10% of the molecules misfold, but when grown at high temperatures (47°C) this increases approximately 4-fold to 41% misfolded. Credit: Bevilacqua Lab, Penn State
Yamagami explained that in their method, live cells are treated with DMS, then RNA is extracted from the cells and selected by size to isolate the tRNA. For sequencing, DNA copies of the RNA molecules must be made. To do this, small segments of DNA, called primers, are first attached to one end of the RNA molecules, which prevents the loss of any information from the short RNA sequence. DNA copies are then made using a special version of the enzyme, "reverse transcriptase," which is not stopped by chemical modifications to the nucleotides when used under certain conditions, but instead often introduces errors when it comes to a modified nucleotide. The researchers then use these errors to identify which nucleotides are modified—a process called "mutational profiling"—to help with their structure predictions.
"With this method we get highly accurate structure predictions at the level of individual molecules," said Bevilacqua. "This also allows us to determine the abundance of each type of tRNA in the cell and the presence of some natural modifications, which can be important for the efficient production of proteins and the overall health of the cell."
Identification and abundance of natural modifications was worked out by Jacob Sieg, a chemistry graduate student in the Bevilacqua lab, through experiments in the Penn State Huck Institutes of the Life Sciences Metabolomics Core Facility.
The researchers performed these experiments in bacterial cells that were cultured in normal temperatures, in cells that were cultured at high temperature (heat stress) and in cells that were given a short burst of high temperature (heat shock). In both heat stress and shock conditions several types of tRNA molecules misfolded and the relative abundance and shape of different tRNAs changed.
"The concept that even a highly structured RNA such as tRNA is conformationally responsive to environmental conditions is an exciting finding from this study," said Sarah M. Assmann, Waller Professor of Biology and an author of the study. "It expands our basic understanding of how cells respond to their environment and stress and this knowledge could help inform the development of novel interventions."
The new method, called "tRNA structure-seq," could be used to study the structure of small RNAs related to human diseases and is not limited to tRNA.
"Seeing how tRNA molecules respond to these stresses and beyond will help us better understand how cells respond to stress and could also help us understand diseases linked to tRNA misfolding," said Bevilacqua. "This new method overcomes the difficulties of working with small, highly modified RNA molecules and can be applied to any organism and to other small RNAs."
More information: Ryota Yamagami et al, Genome-wide analysis of the in vivo tRNA structurome reveals RNA structural and modification dynamics under heat stress, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2201237119
Journal information: Proceedings of the National Academy of Sciences
Provided by Pennsylvania State University