Expanded role for calcium channels in T-cells
A subunit of voltage-gated calcium channels was found to have a functional role in T-cells, according to a Northwestern Medicine study published in Nature Communications.
The findings add to a growing body of knowledge about T-cell activation, and could aid future efforts to modulate immune system activation for a variety of conditions, according to Murali Prakriya, Ph.D., the Magerstadt Professor of Pharmacology and co-senior author of the study.
"Once we have a better understanding of what controls T-cell activation, then we can design approaches to control immune response, both in situations where it is good and where it's bad," said Prakriya, who is also a professor of Medicine in the Division of Allergy and Immunology and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
T-cells are a major component of the body's reactive immune response, and are activated by calcium signaling in response to stimuli such as an invasion by foreign bacteria or viruses.
Upon antigenic stimulation of T-cell receptors, calcium ions enter T-cells by traveling through the Orai1 calcium channel, raising the intracellular calcium concentration. This rise in calcium initiates gene expression, and the production and release of inflammatory mediators that facilitate the hunter-killer functions of T-cells.
In other excitatory cells, such as neurons, voltage-gated calcium channels—distinct from Orai1—play a major role in cellular activation. Some research has revealed signs that voltage-gated calcium channels may also be active in T-cells, but evidence so far remained inconclusive, according to Prakriya.
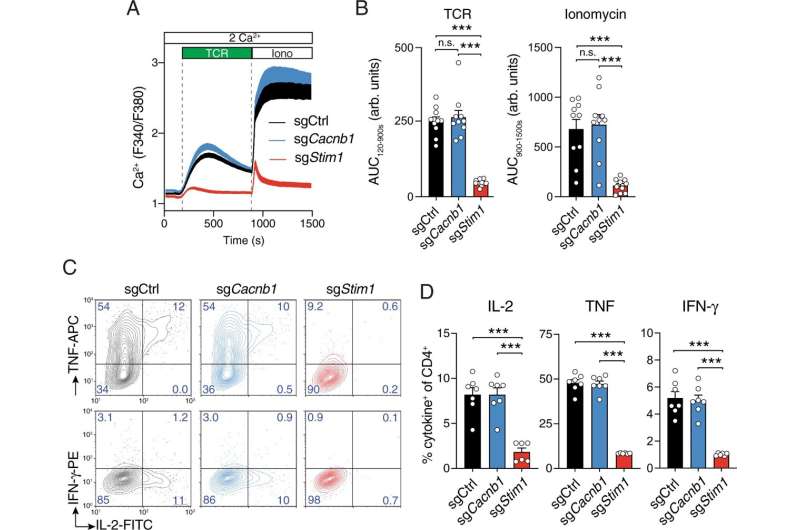
In the current study, investigators examined T-cells isolated from both human and mouse tissue, activating the cells with antigens and looking for signs of voltage-gated calcium channels activation and expression of RNA and proteins. Using a variety of approaches, including simultaneous patch-clamp and calcium imaging techniques, they found no evidence for the presence of functional voltage-gated calcium channels in human and mouse T-cells. Curiously, voltage-gate calcium channel proteins were expressed at the RNA level in the T-cells, but no functional proteins were produced. Instead, these RNA transcripts were truncated and degraded, missing certain key sections that are necessary for channel function.
No functional voltage-gated calcium signaling was detected even in T-cells from human patients with a loss of function mutation in Orai1, which causes an immunodeficiency, indicating that loss of Orai1 signaling does not induce compensatory voltage-gated calcium currents or calcium signals, Prakirya said.
The investigators then took a more granular approach, using gene editing to selectively eliminate certain subunits of voltage-gated calcium channels. These subunits—named alpha and beta—form pores through which calcium ions may flow, but don't have all the functionality of a larger calcium channel.
While the alpha subunit is dispensable—the T-cells activate just fine in response to calcium without it—the beta subunit is required for proper activation, the investigators found.
"These beta subunits seem to have an unknown role in controlling T-cell activation that appears unrelated to their traditional role in modulating the function of the voltage-gated channel pore," Prakriya said. "This role needs to be better understood."
In the future, better control of T-cell activation could be helpful in correcting an overactive inflammation, as seen in autoimmune diseases, or in bolstering the body's defenses against bacteria and viruses.
"Understanding the signaling network that controls T-cells is a fertile area for drug discovery as newer approaches to modulate T-cell responses can be employed for controlling inflammatory and allergic diseases," Prakriya said.
More information: Serap Erdogmus et al, Cavβ1 regulates T cell expansion and apoptosis independently of voltage-gated Ca2+ channel function, Nature Communications (2022). DOI: 10.1038/s41467-022-29725-3
Journal information: Nature Communications
Provided by Northwestern University