Researchers discuss 'WRNing' for the right DNA repair pathway choice
A new editorial paper titled "WRNing for the right DNA repair pathway choice" has been published in Aging .
Premature aging diseases, also called "progeroid syndrome," display signs and features of normal aging in early life, ultimately leading to premature death. Although progeroid syndromes do not perfectly mimic chronological aging, they can be excellent model systems to study characteristics of normal aging.
Werner syndrome (WS) is one of the rare autosomal recessive progeroid syndromes, characterized by accelerated aging. WRN is suggested to play a central role in maintaining genome stability and rapidly recruits to the DNA damage sites to take part in DNA repair, including base excision DNA repair (BER), classical/alternative non-homologous end joining (NHEJ), homologous recombination (HR), and replication re-start after DNA damage.
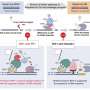
WRN makes critical DNA-repair pathway choices between classical and alternative NHEJs. In addition to its key role in NHEJ, WRN has been suggested to also participate in HR. However, how it regulates the pathway choice between NHEJ and HR is still unclear.
Jong-Hyuk Lee, Deborah L. Croteau and Vilhelm A. Bohr, from the National Institutes of Health's National Institute on Aging, authored a new editorial paper about findings from their recent study.
"In a recent paper, we showed that CDK2 phosphorylating WRN on serine residue 426 is critical for WRN to make its DNA double strand break (DSB) repair pathway choice between NHEJ and HR [4]," they note.
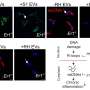
Abnormal DSB recruitment, altered interaction with RPA, strand annealing activity, and DSB repair activities were observed when cells were forced to express WRN engineered to mimic the unphosphorylated or phosphorylation state at serine 426.
The authors observe, "These findings, along with the previous discovered role in NHEJ pathway choice, move our understandings one step closer to the true nature of genomic instability that lies within WS."
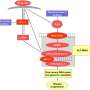
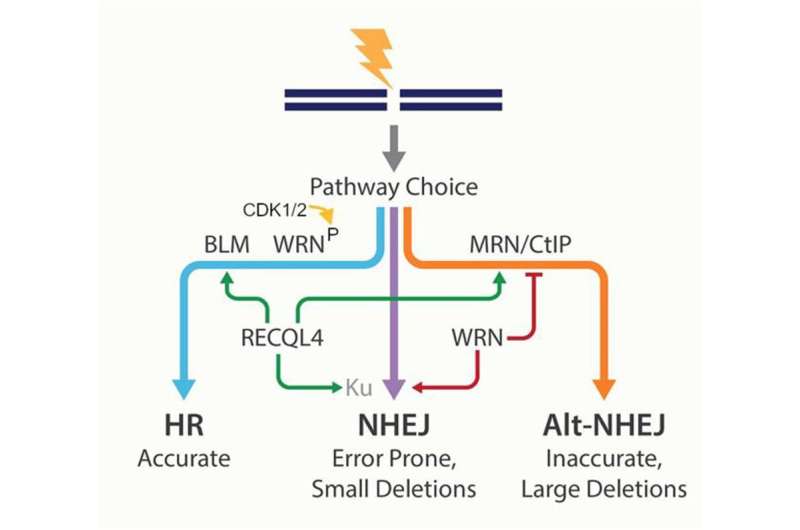
Interestingly, another RECQL family member, RECQL4 has also been identified as one of the crucial decision-makers during DSB repair pathway choice. A graphic representation of RecQs in DSB pathway choice is shown in the image above.
Notably, primary fibroblast cells show similarly increased persistent DNA damage after WRN or RECQL4 knockdown, and CDK regulatory mechanisms on WRN and RECQL4 have functional similarities in DSB response.
"It is thus conceivable that investigating the cooperative roles of WRN and RECQL4 in DSB pathway choice should be a future goal for DNA repair and aging research," the authors conclude.
More information: Jong-Hyuk Lee et al, WRNing for the right DNA repair pathway choice, Aging (2022). DOI: 10.18632/aging.204120
Provided by Impact Journals